Chest wall surgical stabilization after thoracic trauma: indications and techniques
Introduction
The increase in work and road traffic accident has made chest wall traumas very relevant (1,2). This pathological situation is, often, characterized by flail chest, chest wall deformity with displaced rib edges, rib or sternal fractures with nonunion, determining a retraction of the thorax and disability (3,4). Conservative treatment consists in a better control of pain and in a careful bronchial cleaning, up to prolonged mechanical ventilation. This method, in case of flail chest with paradoxical movements of the rib segments or of sternal fracture with overlap of the stumps and/or lesion of the costal cartilages, ensures an internal pneumatic stabilization (5). The main limitation of this technique is related to the risk of ventilator acquired pneumonia, inability to complete reduce the deformity and, lastly, the impossibility of weaning from the ventilator (6). Surgical stabilization adopted once concomitant soft tissue, bone, spine or cranial injuries are resolved, ensures an effective sternocostal stiffness and a physiological mobility of the chest, favouring a valid postoperative respiratory rehabilitation. The chest wall trauma can also determine an additional pathological condition often linked to the common use of seat belts, requiring invasive treatment: the dislocation between the manubrium and the body of the sternum. Restoration of the anatomic structural continuity and alignment of the sternum allows a rapid functional recovery, through the improvement of the respiratory mechanics and lung volumes. The purpose of the study was to evaluate the different surgical methods of sternocostal osteosynthesis, highlighting tips and tricks of techniques.
Flail chest
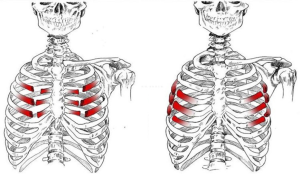
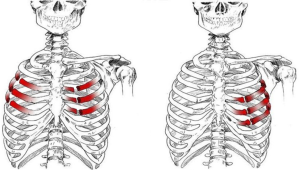
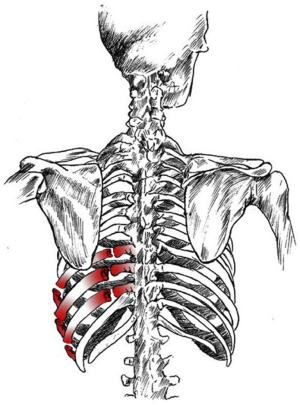
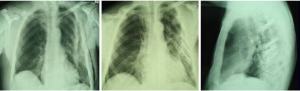
Couraud et al. (7) defined it as “a wall segment disjointed from the rib cage”. The definition of “flail chest” makes a clear reference to the paradoxical movement of the chest wall due to freely movable parietal strip (8,9). Considering the literature, we can define the “flail chest” as a loss of parietal stability following the segmental fracture of 2 or more neighbouring ribs, along with a gradual reduction of lung volumes and deterioration of lung function. Topographically it can be classified as: (I) anterior, with fracture foci regarding only the bone component or the bilateral costochondral joints, usually associated to a lesion of the sternum (Figure 1); (II) lateral or anterolateral, with fracture foci localised both in the bone and cartilage matrix of the ribs (Figure 2); (III) posterior or posterolateral, that rarely require surgical consolidation as parietal stiffness is guaranteed by the scapula and the muscles articulated on the chest (Figure 3); complex, characterized by multiple foci of fracture along the ribs. The type of flail chest dictates the choice of approach that should be adopted before the spontaneous consolidation of the stumps (within the first 4–5 days following the trauma). Three are the options: (I) thoracotomy, with a large section of the latissimus dorsi and serratus anterior. This access provides a wide exposure of the surgical field, dominating pleural cavity and allowing treatment of any concomitant injuries (lung or esophageal lacerations, diaphragmatic, tracheobronchial or vascular ruptures); (II) axillary “muscle-sparing” mini-thoracotomy, indicated in the case of unilateral rib fractures with reduced size and localization; (III) longitudinal suprasternal incision with dissection of the pectoralis major muscle beams on the midline, allows the stabilization of the bilateral rib fractures located in the anterior and lateral costal arches.
Techniques of chest wall surgical stabilization
Flail chest correction carried out with suspension methods of the chest or osteosynthesis implantable devices.
External Traction
It is an obsolete method (10-12), consisting in the perpendicular traction of the floating ribs to the tie-rods in order to fix deformity and ensure normal lung ventilation. Traction, whose degree and duration are linked to both parietal mobility and respiratory insufficiency, can be applied either on soft tissue either or bone component. The first option is performed by the percutaneous introduction of steel wire or metal pin of varying calibre, such as to be positioned tangentially to the external surface of the rib cage and coming out above of the insertion point. Unfortunately, the pulling force on muscles, subcutaneous and skin do not guarantee an acceptable thoracic stability, leading to a progressive tissue section with bleeding and infection risk. The bone component traction requires the dissection of the chosen ribs, usually placed in a central position respect to the flail chest size, making a tunnel between the internal rib surface and the periosteum. The metal device hooks around the ribs in “horseshoe” formation, allowing a constant traction over time. In case of anterior flail chest different techniques were proposed such as a clothes hanger hook (13) or a clamp (14) between the two tables of the sternum, steel wire around the sternum after dissection of retrosternal space or a metal plate fixed to the sternum by two screws with distal hooks in the side edge (12). However, even though the bone traction offers a better performance than the same technique applied to the soft tissues, it also leads to significant complications: (I) the iatrogenic pneumothorax, due to the accidental injury to the parietal pleura which often requires a thoracostomy tube insertion; (II) the sliding of the wire or pin towards the fracture stump, causes progressive bone erosion; (III) bleeding, following the intercostal or internal mammary vessel lesions; (IV) wear of the device.
Kirschner method of osteosynthesis
Currently rarely used, is muscle sparing mini-thoracotomy, both in video-assisted thoracoscopy or percutaneously (15,16). The original technique consists in the introduction of metal pins of appropriate calibre in the central cavity of ribs by drilling the anterior cortex about two centimetres from the lesion. Then, through pronation/supination movements, the device is pushed up to exceed foci fractures, escaping the intact cortex and stabilizing the injury. In order to obtain a greater resistance, it is recommended that the points of entry and exit of the pin must be placed equidistant from each other with respect to the damaged area (Figure 4). Steel wire option offers two possible approaches (17,18): (I) shaping around the fractured ribs to form a metal sleeve; (II) vertical introduction inside the chest, including a rib already stabilised with plate and clavicle or sternum. Kirschner’s technique has several disadvantages: (I) stabilisation of the commuted foci is difficult; (II) sealing is weak, causing wear and fragility of the device; (III) device displacement, determining bleeding for vascular lesion.
Osteosynthesis according to the method of Borelly
The sliding-staples-struts are characterised by a triple component (19,20): (I) the strut, where it is possible to recognize a single hook whose function is to articulate the rib and a “U” shaped mechanism positioned upwardly that represents the sliding plane of the metal plate; (II) the plate, is between 70 and 200 mm in length and not particularly rigid such as to be shaped according to the morphology of the ribs; (III) the straight and angular joints from 15° to 45°, which allow the device to adapt to the rib orientation. The device, shaped before fixation, must be placed perpendicularly to the longitudinal axis of the rib, in order to obtain a good hold (Figure 5). The advantage of this method is linked to the adaptability of the implant, which allows the stabilisation of multiple foci fractures to the replacement of the entire broken rib (Figure 6). Furthermore, Borrelly et al. (21) noticed the reduction in overall mortality of 8% and in length of mechanical ventilation from 5.8 to 2.98 days. The implant characteristics extend its use, with and without meshes or methyl methacrylate prosthesis, in the chest wall reconstruction for large defect following primary or secondary tumour resection (22, 23).
Osteosynthesis with Judet struts
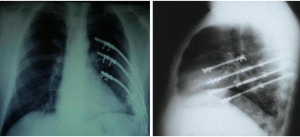
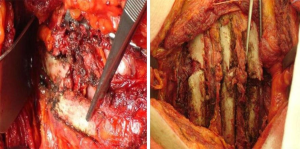
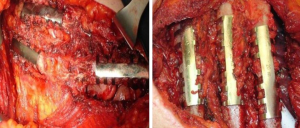
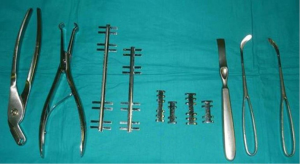
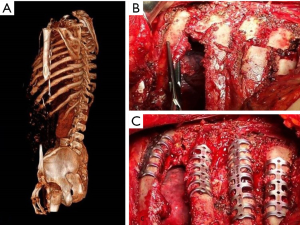
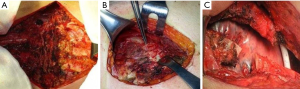
It is a technique described in the seventies (24) but still widely used today because of its excellent functional and morphological results in the unilateral flail chest. Steel struts are characterised by a linear component, variable in length and width (from 12 to 24), with two or three pairs of hooks. The branches of the hooks appear asymmetrical, being respectively at an acute and obtuse angle depending on the fixation at the lower or higher costal margin (Figure 7). The application requires a wide subperiosteal exposure of the outbreaks as well as the preventive reduction of fractures (Figure 8), paying attention to the preservation of the intercostal vascular pedicle in order to avoid necrosis or pseudarthrosis of the stumps. Positioning is carried out by tightening the struts with the specific pliers by means of an abductive movement from the outside towards the inside and a semicircular movement from the bottom to the top (Figure 9). Advantages of this method (25,26) consist in its relative simplicity of execution and the preservation of rib articular mobility despite an excellent stabilization (Figure 10), determining minimal recourse of assisted ventilation and shorter hospitalization. Disadvantages are as follow: 1) the need of a wide posterolateral thoracotomy in order to totally dominate the lesion area; 2) the stabilisation of the single foci fracture for a period of time, increasing the operative time; 3) the possible long-term persistence of pain, if the intercostal nerve is inadvertently pressed between the branches of struts; 4) the spontaneous rupture of the device (Figure 11), requiring immediate removal to avoid vascular lesion. The latter inconvenience seems to be overcome by using the new titanium struts (Figure 12). The use of the Sanchez-Lloret struts (Figure 13) is preferable in the case of multiple comminuted rib fractures (27). This device differs from the Judet struts for their typical straight and not angled claws of the hooks, facilitating their modelling based on the physiological orientation of the ribs.
Osteosynthesis with titanium plate and screws
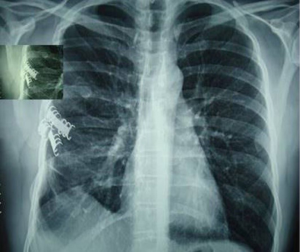
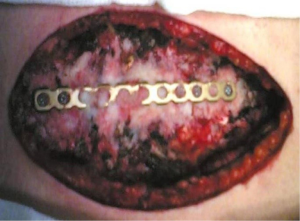
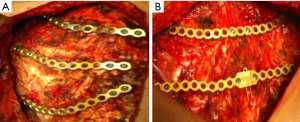
In the last decade, titanium has replaced steel for the low friction coefficient and the high corrosion-thermal-mechanical resistance despite the low density. The use of plate is mainly recommended in anterior, anterolateral and lateral flail chest especially in case of bone loss (Figure 14). Types of plaques and the application techniques are variegated (28-30). In our clinical practice we use threaded screws (3 mm in diameter, 8–18 mm in length) and titanium plates (2.4 mm in thickness, 248.5 mm in length for 30 threaded holes divided into two parts of 15 holes with a central link). Using a special tool, the plates can be modelled based on the morphological characteristics of the lesion (Figure 15); the screws, whose length is measured with accuracy by means of the depth gauge, are fixed by making a hole in the anterior cortex using a drill with an appropriate locking mechanism so as not to exceed the posterior cortex. Anterior flail chest can be successfully treated by means of a single 30 holes plate, articulated centrally with respect to the longitudinal diameter of the defect. In order to ensure a symmetrical respiratory dynamics it is crucial that the two parts of 15 holes are bilaterally equidistant from the foci fractures; this involves firstly the central link fixation of plate to the sternum and subsequently the application of its distal ends over the stumps, on an undamaged segment of rib. In case of anterolateral or lateral flail chest the osteosynthesis with plate allows restoration of the normal thoracic continuity and chest wall stiffness (Figure 16). Despite the undoubted advantages of the method (running fast, safe and easily with an excellent stability of the rib cage) and the minimal complications (reduction of rib mobility or infection requiring the removal of device) there is still a lack of consensus in using titanium plate. Landercasper et al. (31), in 32 flail chest patients not surgically treated, showed that the 25% complained of chest restriction, 50% of persistent chest pain and 57% of spirometry abnormality at 5 year follow-up. Marasco et al. (32), studying 23 patients surgically and 23 patients conservatively treated, revealed better findings with the invasive approach in: (I) total intensive care unit stay (324 vs. 448 hours, P=0.03); (II) hospitalization (20 vs. 25 days, P=0.24); c) cost saving ($14,443 per operated patient).
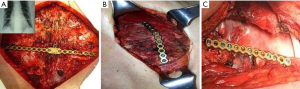
Other techniques of osteosynthesis
Debates on the surgical approach to flail chest led to the development of methods, which still require extensive clinical confirmation. Bibas et al. (33) have proposed the use of Marlex mesh, fixed through polypropylene zero to totally cover the site of the lesion and coated with 40 mL of methyl methacrylate, whose polymerisation determines the invasion of the adjacent bone and muscles. Advantages consist in the easy supply of the devices, the reduction of operative time and the minimal tendency to infection. Mayberry et al. (34) have treated 10 flail chest patients by resorbable polylactide plate, shaped accordingly to the rib morphology by heating it to 60 degrees for 15 seconds. Disadvantages were: (I) the impossibility of weaning from mechanical ventilation (5 patients); (II) pain with thoracic instability (4 patients); (III) large chest wall defect (1 patient). The main advantages of this method compared to the plate technique consist in a more rapid and durable healing as well as the unnecessary removing of the device.
Flail chest
Flail chest determines a mixed respiratory insufficiency due to oxygen transfer alteration and hypoventilation with carbon dioxide retention. We tried to correct this pathological alteration by the association between conservative treatment and surgical approach. Advantages and disadvantages of different methods of osteosynthesis are already described in the respective paragraphs. Two aspects deserve to be further investigated: a) the thoracic analgesia; b) the steroid treatment for pulmonary contusion. Thoracic epidural analgesia (TEA) is the gold standard to providing adequate pain relief (35) although it is used rather than intercostal or paravertebral nerve blocks or patient-centered analgesia. This underutilization is due to technical failures (up to 30%) and experience or skills of the anesthesiologist (36). Paravertebral analgesia may be equivalent to epidural analgesia and may be appropriate when epidural is contraindicated. Currently, dexmedetomidine has been proposed because of high selective α-2 adrenoceptor agonists showing analgesic, sedative, sympatholytic and amnestic properties without respiratory depression (37,38). Pulmonary contusion due to blunt chest trauma leads to an early activation of the oxidant-antioxidant cascade, a delayed pulmonary capillary leakage, an elevation both in thromboxane and prostacyclin values; this reaction precedes the progressive bilateral neutrophil infiltration. The complications and risks versus benefit of steroid use have been widely investigated (39). Boybeyi et al. (40), studying the dimethyl sulfoxide and dexamethasone intraperitoneally administered in experimental pulmonary contused models, noticed that the first prevents further pulmonary injury decreasing both neutrophil infiltration and endothelial injury while the second might have a role in the development of inflammation. Ocalan et al. (41), analyzing the budesonide inhalatory effects on pulmonary contusion in 55 rats, showed an improvement of SatO2 and PaO2 compatible with a minimization of inflammatory pulmonary reactions.
Isolated sternal fracture and manubriosternal dislocation
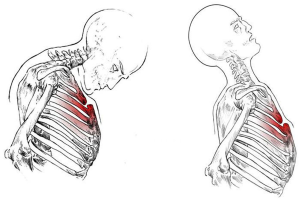
The first case of surgical correction of the sternal fracture was described by McKim in 1943 (42). However, the randomized studies regarding the indications and techniques for traumatic sternal lesion stabilization are few and incomplete despite the considerable increase in injuries. Knobloch et al. (43) revealed 0.64% of sternal fractures in 42,055 traumatized patients as well as the correlation between injury severity score (r2=0.92) and maximal abbreviated injury scale (r2=0.81) and the deceleration velocity. Harston et al. (44), reviewing over 20 years of literature, displayed 52 patients with plates and 24 with wires surgically treated for sternal fracture, highlighting the need for a shared management especially in absence of concomitant injuries. In fact, the associated soft tissue, chest wall, spine and cranium lesions showed mortality between 25% and 45% (45). Also, we have noticed (46) in 65 patients with an isolated sternal fracture and a manubriosternal dislocation underwent surgical approach the quality-of-life improvement, the safe intraoperative and postoperative management using titanium plate compared with steel plate and steel wire. Surgical stabilization of isolated sternal fracture without underlying injuries is still particularly debated. Mayberry et al. (47) have found three key points: (I) the presence of a sternal deformity; (II) the sternal continuity loss for a period more than six weeks; (III) the persistence of chest pain, between two and eight weeks after trauma for the majority of surveyed surgeons. Based on our experience we suggested to add the oblique stumps and unstable nonunion of the sternum, with the overlap and mobility of the outbreak fractures. The indirect mechanism of trauma determines the hyperextension of the cervical and dorsal spine, or the hyperflexion of the head on the chest (Figure 17), or the sudden contraction of the abdominal and sternocleidomastoid muscles. Morphological characteristics of the sternal dislocation depend on the type of traumatic event, showing the posterior (Type 1: direct trauma) or anterior luxation (Type 2: indirect trauma) of the sternal body in respect to the manubrium. In this condition, the invasive approach is indicated in the case of respiratory failure due to a morphological alteration of the rib cage, kyphosis of the cervicothoracic spine with neck stiffness and pain associated with a serious esthetic damage. Surgical osteosynthesis of traumatic sternal lesions, the purpose of which is the restoration of the normal dynamic ventilation, is frequently pursued through metal wires or rigid plate and screws.
Osteosynthesis with metal wires
It is not an easy technique but still widely in use (47,48), although clinical results are questionable. Method consists in the midline section of the pectoralis major muscle sternal beams, subperiosteal exposure of the fracture or luxation foci, dissection of the retrosternal and intercostal spaces bilaterally in order to stabilize the stumps by means of U-shaped or X-shaped stitches in the full thickness of the bone. The congruous dissection of the surgical site and surrounding structures prevents an abnormal or wrong positioning of the wire (Figure 18); the patient may incur in a high risk of vascular lesions facing a poor sternal stability.
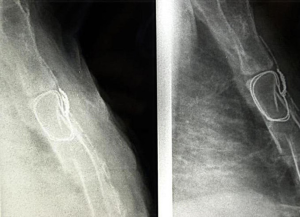
Osteosynthesis with plate and screws
Different methods supported by a wide material availability have been proposed in sternal surgical stabilization (49-51). In our clinical practice we used both metal and titanium plates, characterised by an extremely easy fixation technique.
Isolated Sternal Fracture
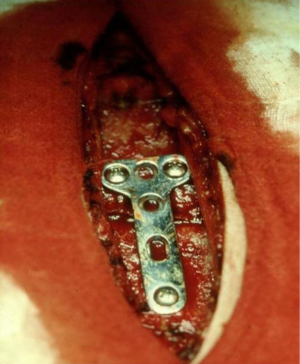
The steel plates, no longer commercially available, are T-shaped. After section of the pectoralis major muscle sternal beams and subperiosteal exposure of the fracture site, the plate is fixed to the sternum by 3–5 threaded screws inserted into the anterior cortical margin and anchored to the posterior cortical in order to lift and stabilize the stumps (Figure 19). While this method allows an excellent surgical correction, three are the drawbacks: (I) difficult shaping of the plate, particularly rigid; (II) choice of the screw on the basis of the surgeon’s experience and not through the clear assessment of the required length; (III) displacement of the plate and screws in the suprasternal plane or between the beams of the major pectoralis muscle. These difficulties seem to be overcome with the new titanium plate, which can be easily shaped according to the morphological aspects of the lesion. The threaded holes of the plate allow the simultaneous anchoring of the threaded screws both to the sternum and the plate itself, inhibiting the likelihood of the displacement of the implant. Also, the use of a depth gauge able to calculate the distance between the two sternal cortical margins, ensures to the ability of objectively establishing the exact length of the screws to be employed. Gallo et al. (52) have set out three technical principles to be followed in the case of chronic traumatic sternal fracture: (I) the removal of the abnormal bone callus; (II) the internal fixation using rigid plates; (III) the autologous bone graft. In our experience, we have never carried out the bone transplant. If the loss of substance determined a solution of continuity of the sternum, we fix the implant with demineralized bone matrix (Figure 20).
Manubriosternal dislocation
After a longitudinal incision of the skin and subcutaneous tissue, the pectoralis major beams are dissected from the second to the fifth rib bilaterally for 3–4 cm (46). The subperiosteal dissection of the sternum, the excision of the manubriosternal synchondrosis already damaged by the traumatic event and the cuneiform chondrectomy of the third and fourth rib cartilage bilaterally, in order to align the manubrium and the body of the sternum, were carried out. From two to four threaded screws, whose length was precisely established by means of a depth gauge, per side based on the extension of the dislocation foci allow titanium plate fixation with the method already described. The use of the central part of the plate, including fitting U-shaped and needle hooking, ensures the physiological adaptation of the sternum to the respiratory excursions of the chest wall. Whether associating a sternal fracture, the application of the linear ends of the plate allows a better stiffness (Figure 21). Use of demineralized bone matrix can facilitate the bone callus formation due to the osteoinductive activity and the surrounding blood cells compatibility. Two 18 French Spiral drains were placed above the rib cage in order to avoid a blood or serous fluid collection. Advantages of this technique are as follows: (I) excellent stabilization, with preservation of the chest motility; (II) inhibition of serious thoracic spine and cage deformities; (III) reduction of iatrogenic complications; (IV) rapid recovery of respiratory function; (V) shorter duration of hospital stay with reduction in costs; (VI) undeniable aesthetic and antalgic outcomes.
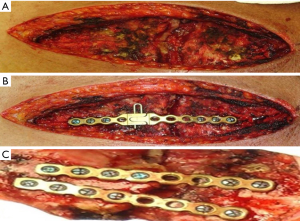
Traumatic sternal lesions
The methods for isolated sternal fracture stabilization are still debated. Different techniques were proposed successfully: (I) wires associated with two threaded pins inserted between the two tables of the sternum, crossing the line of fracture (53); (II) X-shaped plate (54); (III) volar distal radius plate (55). We showed excellent outcomes with linear titanium plate. This device allows: (I) the rigid support of the sternum, inhibiting the abnormal movement of the fracture foci and/or bleeding; (II) the prevention of implant displacement, by means of the screws anchored simultaneously to the plate and sternum; (III) the rapid recovery of respiratory function associated with the pain reduction, determining excellent quality-adjusted life years. Manubriosternal dislocation is not widely treated in literature. We opine that respiratory insufficiency based on ankylosis and chest deformity, kyphosis of rachis with stiff neck, chronic pain syndrome and aesthetic damage lead to surgical fixation. A stapling technique (2 Blount staples) (56) in 1 patient, an angular stable implant (57) in 1 patient, an 8-hole one-third tubular plate (58) in 2 patients and a 3.5/4.0 mm fixed-angle plate (59) in 3 patients were used over time with positive results. In our experience, linear titanium plate allowed to restore the normal motility and rigidity of the chest wall without sequelae. The progressive loss in tension of steel wires determined a morphological alteration of the thorax, pain and anxiety resulting in a poor quality-adjusted life years. Also, we have already demonstrated in 18 patients (46) that titanium implant gained 4 QALYs more than steel wires, justifying costs. The incremental cost-effectiveness ratio, supporting these outcomes, explained that costs should always be proportionate to the obtained health return.
Surgical approach to stabilize the rib cage after trauma must respond to the need of a minor invasiveness, to an easy and quick execution and to an overall safety, avoiding complications or recurrences. In case of flail chest, the use of Judet struts or the rigid titanium plate meets these criteria. Titanium implant is especially recommended if there is a bone loss or multiple foci of fracture, even replacing the ribs totally. In our experience we noticed the clinical advantages resulting from the association of mechanical ventilation and surgical treatment. The shorter duration of ventilatory support is aimed at gas exchange stabilization and acute respiratory distress resolution. The early surgical osteosynthesis of the fracture foci, restoring the respiratory dynamic and the lung function stability, avoids pulmonary and cardiac failures. According to the our experience, the use of linear titanium plate associated or not with demineralized bone matrix is recommended in case of isolated sternal fracture and manubriosternal dislocation. In fact, we noticed an improvement of quality-adjusted life associated with the economic sustainability of intervention. However, we believe that no technique should be subject to prejudice but rather the effectiveness of each method must be assessed in terms of adequate stabilisation and proper ventilation reflecting the anatomical and functional integrity of the chest wall. It is clear that the experience and skill of the surgeon is crucial to obtain excellent results, in order to avoid the restrictions in both the work and social life of the patient.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci, Alan D.L. Sihoe and Benedetta Bedetti) for the series “Open Thoracic Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.05.04). The series “Open Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liman ST, Kuzucu A, Tastepe AI, et al. Chest injury due to blunt trauma. Eur J Cardiothorac Surg 2003;23:374-8. [Crossref] [PubMed]
- Divisi D, Crisci R. Use of demineralized bone matrix and plate for sternal stabilization after traumatic dislocation. Gen Thorac Cardiovasc Surg 2011;59:52-6. [Crossref] [PubMed]
- Khoriati AA, Rajakulasingam R, Shah R. Sternal fractures and their management. J Emerg Trauma Shock 2013;6:113-6. [Crossref] [PubMed]
- Lafferty PM, Anavian J, Will RE, et al. Operative treatment of chest wall injuries: indications, technique, and outcomes. J Bone Joint Surg Am 2011;93:97-110. [Crossref] [PubMed]
- Lardinois D, Krueger T, Dusmet M, et al. Pulmonary function testing after operative stabilization of the chest wall for flail chest. Eur J Cardiothorac Surg 2001;20:496-501. [Crossref] [PubMed]
- Tanaka H, Yukioka T, Yamaguti Y, et al. Surgical stabilization or internal pneumatic stabilization? A prospective randomized study of management of severe flail chest patients. J Trauma 2002;52:727-32. [Crossref] [PubMed]
- Couraud L, Bruneteau A, Durandeau A. Flail chest. Therapeutic indications in relation to the site and clinical picture. Ann Chir Thorac Cardiovasc 1973;12:15-8. [PubMed]
- Pettiford BL, Luketich JD, Landreneau RJ. The management of flail chest. Thorac Surg Clin 2007;17:25-33. [Crossref] [PubMed]
- Leinicke JA, Elmore L, Freeman BD, et al. Operative management of rib fractures in the setting of flail chest: a systematic review and meta-analysis. Ann Surg 2013;258:914-21. [Crossref] [PubMed]
- Jones T, Richardson E. Traction on the sternum in the treatment of multiple fractured ribs. Surg Gynecol Obstet 1926;42:283-5.
- Gardner CE Jr. Chest injuries; application of military experience to civilian practice. Surg Clin North Am 1946;26:1082-94. [PubMed]
- Jancovici R, Pons F, Duberez J, et al. Traitement chirurgical des traumatismes thoraciques (II). Encycl Méd Chir (Elsevier, Paris), Techniques chirurgicales – Thorax, 42-445-B, 1997, 22 p.
- Jaslow IA. Skeletal traction in the treatment of multiple fractures of the thoracic cage. Am J Surg 1946;72:753-5. [Crossref] [PubMed]
- Heroy WW, Eggleston FC. A method of skeletal traction applied through the sternum in “steering wheel” injury of the chest. Ann Surg 1951;133:135-8. [Crossref] [PubMed]
- Marcheix B, Brouchet L, Renaud C, et al. Technique de l’ostéosynthèse costale. Encycl Méd Chir (Elsevier, Paris), Techniques chirurgicales – Thorax, 42-473, 2005, 11 p.
- Ahmed Z, Mohyuddin Z. Management of flail chest injury: internal fixation versus endotracheal intubation. J Thorac Cardiovasc Surg 1995;110:1676-80. [Crossref] [PubMed]
- Beltrami V, Martinelli G, Giansante P, et al. An original technique for surgical stabilization of traumatic flail chest. Thorax 1978;33:528-9. [Crossref] [PubMed]
- Mombelloni G, Fabio D. Indirizzi terapeutici dei trauma della parete toracica e del diaframma. In “I Traumi del Torace e dell’Apparato Cardiovascolare” – XXII Congresso Nazionale S.I.C.T. Bari, 10-13 Luglio 1990;9-21.
- Borrelly J, Grosdidier G, Wack B. Traitement chirurgical de l’instabilité pariétale thoracique par l’atelle-agrafe à glissière (AAG). Rev Chir Orthop 1985;71:241-50. [PubMed]
- Borrelly J, Grosdidier G, Wack B. Notre experience de 5 ans d’utilisation d’un nouveau materiel d’ostéosynthèse thoracique: l’attelle-agrafe à glissière (AAG). Ann Chir Thorac Cardiovasc 1985;39:465-70.
- Borrelly J, Aazami MH. New insights into pathophysiology of flail segment: the implications of anterior serratus muscle in parietal failure. Eur J Cardiothorac Surg 2005;28:742-9. [Crossref] [PubMed]
- Divisi D, Ferrera R, Montagna P, et al. Chest wall tumors. Report of 17 cases. Rev Mal Respir 1999;16:369-78. [PubMed]
- Thomas PA, Brouchet L. Prosthetic reconstruction of the chest wall. Thorac Surg Clin 2010;20:551-8. [Crossref] [PubMed]
- Judet R. Costal osteosynthesis. Rev Chir Orthop Reparatrice Appar Mot 1973;59:Suppl 1:334-5. [PubMed]
- Menard A, Testart J, Philippe JM, et al. Treatment of flail chest with Judet’s struts. J Thorac Cardiovasc Surg 1983;86:300-5. [PubMed]
- Bemelman M, Poeze M, Blokhuis TJ, et al. Historic overview of treatment techniques for rib fractures and flail chest. Eur J Trauma Emerg Surg 2010;36:407-15. [Crossref] [PubMed]
- Sánchez-Lloret J, Letang E, Calleja MA, et al. Indication and surgical treatment of the traumatic flail chest syndrome: an original technique. Thorac Cardiovasc Surg 1982;30:294-7. [Crossref] [PubMed]
- Engel C, Krieg JC, Madey SM, et al. Operative chest wall fixation with osteosynthesis plates. J Trauma 2005;58:181-6. [Crossref] [PubMed]
- Mouton W, Lardinois D, Ferrer M, et al. Long-term follow-up of patients with operative stabilization of a flail chest. Thorac Cardiovasc Surg 1997;45:242-4. [Crossref] [PubMed]
- Coonar AS, Qureshi N, Smith I, et al. A novel titanium rib bridge system for chest wall reconstruction. Ann Thorac Surg 2009;87:e46-e48. [Crossref] [PubMed]
- Landercasper J, Cogbill T, Lindesmith L. Long-term disability after flail chest injury. J Trauma 1984;24:410-4. [Crossref] [PubMed]
- Marasco SF, Davies AR, Cooper J, et al. Prospective randomized controlled trial of operative rib fixation in traumatic flail chest. J Am Coll Surg 2013;216:924-32. [Crossref] [PubMed]
- Bibas BJ, Bibas RA. Operative stabilization of flail chest using a prosthetic mesh and methylmethacrylate. Eur J Cardiothorac Surg 2006;29:1064-6. [Crossref] [PubMed]
- Mayberry JC, Terhes JT, Ellis TJ, et al. Absorbable plates for rib fracture repair:preliminary experience. J Trauma 2003;55:835-9. [Crossref] [PubMed]
- Simon BJ, Cushman J, Barraco R, et al. Pain management guidelines for blunt thoracic trauma. J Trauma 2005;59:1256-67. [Crossref] [PubMed]
- Grau T, Leipold R, Conradi R, et al. Ultrasonography and peridural anesthesia. Technical possibilities and limitations of ultrasonic examination of the epidural space. Anaesthesist 2001;50:94-101. [Crossref] [PubMed]
- Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol 2008;21:457-61. [Crossref] [PubMed]
- Arain SR, Ruehlow RM, Uhrich TD, et al. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg 2004;98:153-8. [Crossref] [PubMed]
- Simon B, Ebert J, Bokhari F, et al. Management of pulmonary contusion and flail chest: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg 2012;73:S351-361. [Crossref] [PubMed]
- Boybeyi O, Bakar B, Aslan MK, et al. Evaluation of dimethyl sulfoxide and dexamethasone on pulmonary contusion in experimental blunt thoracic trauma. Thorac Cardiovasc Surg 2014;62:710-5. [PubMed]
- Ocalan K, Solak O, Esme H, et al. Efficacy of budesonide and interleukin-10 in an experimental rat model with isolated bilateral pulmonary contusion created by blunt thoracic trauma. Eur J Cardiothorac Surg 2013;43:163-7. [Crossref] [PubMed]
- McKim LH. A method of fixation for fractures of the sternum. Ann Surg 1943;118:158-60. [Crossref]
- Knobloch K, Wagner S, Haasper C, et al. Sternal fractures are frequent among polytraumatised patients following high deceleration velocities in a severe vehicle crash. Injury 2008;39:36-43. [Crossref] [PubMed]
- Harston A, Roberts C. Fixation of sternal fractures: a systematic review. J Trauma 2011;71:1875-9. [Crossref] [PubMed]
- Khoriati AA, Rajakulasingam R, Shah R. Sternal fractures and their management. J Emerg Trauma Shock 2013;6:113-6. [Crossref] [PubMed]
- Divisi D, Di Leonardo G, Crisci R. Surgical management of traumatic isolated sternal fracture and manubriosternal dislocation. J Trauma Acute Care Surg 2013;75:824-9. [Crossref] [PubMed]
- Mayberry JC, Ham LB, Schipper PH, et al. Surveyed opinion of American Traum Orthopedic, and Thoracic Surgeons on rib and sternal fracture repair. J Trauma 2009;66:875-9. [Crossref] [PubMed]
- Molnar TF. Surgical management of chest wall trauma. Thorac Surg Clin 2010;20:475-85. [Crossref] [PubMed]
- Song DH, Lohman RF, Renucci JD, et al. Primary plating in high-risk patients prevents mediastinitis. Eur J Cardiothorac Surg 2004;26:367-72. [Crossref] [PubMed]
- Voss B, Bauernschmitt R, Brockmann G, et al. Complicated sternal dehiscence: reconstruction with plates, cables, and cannulated screws. Ann Thorac Surg 2009;87:1304-6. [Crossref] [PubMed]
- Raman J, Straus D, Song DH. Rigid plate fixation of the sternum. Ann Thorac Surg 2007;84:1056-8. [Crossref] [PubMed]
- Gallo DR, Lett ED, Conner WC. Surgical repair of chronic traumatic sternal fracture. Ann Thorac Surg 2006;81:726-8. [Crossref] [PubMed]
- Molina JE. Evaluation and operative technique to repair isolated sternal fractures. J Thorac Cardiovasc Surg 2005;130:445-8. [Crossref] [PubMed]
- Chou SS, Sena MJ, Wong MS. Use of sternalock plating system in acute treatment of unstable traumatic sternal fractures. Ann Thorac Surg 2011;91:597-9. [Crossref] [PubMed]
- Ergene G, Tulay CM, Anasiz H. Sternal fixation with nonspecific plate. Ann Thorac Cardiovasc Surg 2013;19:364-7. [Crossref] [PubMed]
- El Ibrahimi A, Smahi M, Shimi M, et al. Traumatic manubriosternal dislocation: A new method of stabilization postreduction. J Emerg Trauma Shock 2011;4:317-9. [Crossref] [PubMed]
- Nijs S, Broos PL. Sterno-manubrial dislocation in a 9-year-old gymnast. Acta Chir Belg 2005;105:422-4. [Crossref] [PubMed]
- Kälicke T, Frangen TM, Müller EJ, et al. Traumatic manubriosternal dislocation. Arch Orthop Trauma Surg 2006;126:411-6. [Crossref] [PubMed]
- Gloyer MA, Frei HC, Hotz TK, et al. Osteosynthesis of traumatic manubriosternal dislocations and sternal fractures with a 3.5/4.0 mm fixed-angle plate (LCP). Arch Orthop Trauma Surg 2011;131:1261-6. [Crossref] [PubMed]
Cite this article as: Crisci R, Divisi D. Chest wall surgical stabilization after thoracic trauma: indications and techniques. Shanghai Chest 2017;1:5.