Left side sleeves
Introduction
Sleeve bronchial lobectomy (SL) is defined as the resection of a pulmonary lobe associated with resection and reconstruction of the bronchus and, in some instances, depending on the site of the infiltration of the neoplasm, it can be associated with resection and reconstruction of the pulmonary artery (PA).
The principles and the basic technique of these particular reconstructive procedures were established in the 1940s and 1950s by Prince-Thomas and Johnson and promoted by the improved definition of the pulmonary anatomy.
Since the mid 1970s, conservative techniques (sleeve lobectomies) have been widely accepted in the management of lung cancer with best long-term results compared to those reported following pneumonectomy (1-3).
The association of SL to PA resection and reconstruction (double sleeve) has been less widely used compared to bronchial reconstruction alone, probably due to the discouraging results in terms of complications and to the heterogeneity of populations and varying surgical techniques reported in published studies (4-8). To date there are few reports on broncho-angioplastic intervention (BAI) in literature. This may be due to the particularly risk patients, to the surgical intervention which may be complicated, and to the apparently dismal long-term results (4-8). However, these surgical procedures remain technically demanding and have finally gain a definitive role in the management of lung cancer.
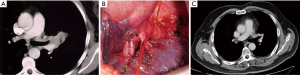
Indications for BAI in patients with lung cancer are: (I) when a tumor infiltrates the origin of the lobar bronchus (usually the upper one) and the PA or the origin of the lobar branches of the PA (Figure 1A,B), a condition that debar simple lobectomy but does not require pneumonectomy; (II) in case of N1 (hilar) lymph node infiltration of the bronchus and the PA (Figure 1C) or its branches, as is often the case in the left upper lobe tumors; (III) after induction treatments when unremovable fibrotic tissue or residual tumor is simultaneously embedded in the bronchus and PA; (IV) to avoid pneumonectomy in patients with compromised cardiac and/or pulmonary function. Our policy in case of locally advanced lung cancer was to preserve as much lung parenchyma as technically and oncologically possible. Thus, arterial sleeve resection was carried out when possible and not only in cases of respiratory impairment contraindicating pneumonectomy. Sometimes a BAI is associated with an upper bilobectomy due to the tumor or nodal infiltration at the level of intermediate bronchus.
PA can be infiltrated by the tumor to various levels, from partial infiltration to a more extensive and even circumferential involvement. In case of partial involvement, a partial resection with suture of the PA is indicated; in case of extended involvement, the reconstruction of the PA may be made by using pericardial patch, end-to-end anastomosis, or by the interposition of a prosthetic conduit.
Preoperatively, patient undergo standardized diagnostic and staging procedures. Key point in the preoperative staging are chest/upper abdomen computed tomography (CT) scan, positron emission tomography scan, and bronchoscopy. This latter examination may easily ascertain the infiltration of the origin of the lobar bronchus and allows to obtain specimens for cyto-or histopathology. If at the CT scan are present enlarged mediastinal lymph nodes (paratracheal or subcarinal regions) greater than 1 cm or PET-positive, or both, the patient underwent endobronchial ultrasound-guided transbronchial needle aspiration. R2 or L2 node involvement is an absolute contraindication to surgery even in case of a partial or complete response to induction chemotherapy. Adenopathy in mediastinal station R4 is not an absolute contraindication to surgery; in these cases, patients receive preoperative chemotherapy with the intent to treat systemic microscopic metastases and to identify progressive disease, which would contraindicate surgical resection. At the end of the scheduled induction treatment, patients underwent total-body CT scan and PET scan for re-stage the disease.
Preoperative pathologic confirmation of PA infiltration is impossible to obtain. It may be suspected and assessed by CT scan and eventually angiography and magnetic resonance imaging scans, but the need of resection is usually made intraoperatively.
Preoperative cardiac evaluation including echography should be performed in every case. Sometimes, a stress electrocardiography, myocardial scintigraphy, or coronary angiography may be performed in case of presence of cardiovascular symptoms. Respiratory function is assessed by blood gas analysis, spirometry with the evaluation of carbon monoxide lung diffusing capacity, and lung perfusion scan.
Operative technique
Preparation
The patient is intubated with a double-lumen for a one-lung ventilation. Patient is monitored with continuous arterial and venous pressure measurements. The patient is placed in the lateral position with the back moved towards the edge of the table. The sterile field include the axilla, anterior chest across the sternum and neck, and the spine.
Exposition
Surgical team is placed as follows: the surgeon is placed to the patient’s back; the first assistant across the table, and the second assistant near to the surgeon. The intervention is performed trough a muscle sparing lateral thoracotomy. The incision is made in the fourth or fifth right intercostal space from the anterior edge of the latissimus dorsi muscle to the posterior edge of the pectoralis major (Figure 2). In females, the axillo-mammary fold is cosmetically preferred.
Operation
The first step of the intervention is the pleural cavity exploration and then a complete dissection of the fissure. Mediastinal lymph node dissection is routinely performed at the beginning of the procedure trying not to devascularise the bronchus beyond the line of section. When the distal parenchyma is free of tumor and/or invasive growth lymph nodes, a sleeve resection is performed. The main and distal bronchus are isolated; after the resection of the bronchial segment concerned and severance of the pulmonary ligament for mobilization, the corresponding ands of the bronchus can usually be readily brought together. Resection is considered complete if resection margins were free of tumor at frozen section.
The anastomosis between the main left bronchus and the left lower bronchus is performed with two running sutures with a non-absorbable monofilament (3/0 polypropylene; Ethicon, Inc, Sommerville, NJ). The stitches are positioned at the far side of the cartilaginous wall and subsequently fixed. Afterwards, two running sutures, first passed and secondarily tied, are performed in order to obtain a telescopic anastomosis (Figure 3A).
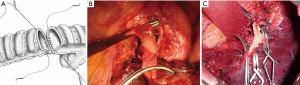
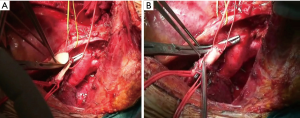
The surgical technique for pulmonary artery resection included the following steps: the first step is to isolate the main PA which is usually controlled proximally, extra-pericardically (Figure 3A); in some instances (involvement of the initial portion of the PA), the temporary proximal occlusion is done intra-pericardically. On the left side, the problems of dissection are less, since the PA can be readily mobilized after severance of the ligamentum arteriosus and the pericardial fold avoiding left recurrent laryngeal nerve damage. Centrally, the main PA must be carefully occluded with a soft atraumatic vascular clamp in order to avoid intimal injuries of the frequently sclerotic vessels. Distal control of the PA is usually obtained in the pulmonary fissure with another vascular clamp (Figure 3A) and/or bulldog vascular clamp allowing sufficient room for the operating surgeon (Figure 3B). Sometimes, to have a more room for the surgical resection and reconstruction and to prevent back-bleeding, it is possible to clamp the vein of the other lobe. The pulmonary vein of the lobe which should be resected is isolated and then sectioned using a vascular stapler. Systemic anticoagulation is initiated (3,000 to 5,000 IU heparin sodium) and not reversed by protamine at the end of the procedure. Thereafter, resection of the targeted lobe is carried out en bloc with the invaded portion of the PA. The pulmonary lobe containing the tumor is removed from the operative field offering to the surgeon more room for complete the work. Frozen section of both the margins (bronchus and PA) should be sent. If a bronchial positive margin is found at the frozen section, a pneumonectomy is performed whenever functionally tolerable.
In all cases of the bronchial and vascular reconstruction, we prefer to perform the arterial reconstruction first which decrease the clamp time, followed by the bronchial reconstruction.
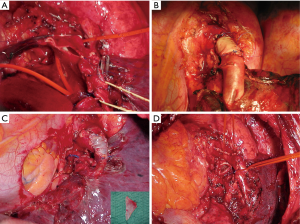
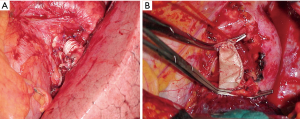
The resected PA is now evaluated to determine the type of arterial reconstruction: if the residual arterial calibre is ≥50%, a direct suture is carried out using 5-0 or 6/0 polypropylene suture (Figure 4A,B). Otherwise, application of an autologous (Figure 4C) or heterologous (bovine) pericardial patch is used (Figure 4D). After the resection of the upper lobe and the PA, a longitudinal arterial defect ensues (Figure 5A). A patch is held in place by two stay sutures. The shape and size of the patch should be tailored to the resected portion of the PA. The patch is sutured with 5/0 or 6/0 non-absorbable monofilament after adequately trimming it (Figure 5B). In some circumstances, a sleeve resection of the PA is required with end-to-end anastomosis avoiding irregular and uneven edges of the transacted PA facilitates proper placement of the stitches. In this case, calibre discrepancy is compensated by elasticity of the wall of the vessel. If a long circumferential resection of the PA is required the interposition of a conduit (autologous or heterologous) is performed. This may occur in the case of extended defects in which end-to-end anastomosis is not possible because the PA is extensively infiltrated by the tumor, and the vascular edges remain far away after the bronchial sleeve resection. PTFE grafts (Figure 6A) used for PA reconstruction have some risks including the risk of infection, and thrombosis, and the need for postoperative anticoagulation. Moreover, in some instances, the autologous pericardium may be insufficient to construct a long tube. For this reason, we have suggested the use of a bovine custom-made pericardial prosthesis (Figure 6B) which is resistant to the infection, it is easy to handling, and it does not need for anticoagulation. In this case, the proximal anastomosis between the PA and the custom-made pericardial prosthesis is performed first while the distal one is performed last. Suturing is carried out using 5-0 polypropylene suture. Just before tying the arterial suture, the distal clamp is taken off from the PA or the pulmonary vein to help remove the air from the PA. Then the proximal vascular clamp is removed from the PA to ensure haemostasis of the sutured line. It is crucial the absence of tension on the graft. Moreover, graft redundancy should be avoided because it could cause the torsion or kinking of the graft.
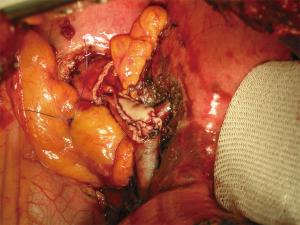
After completion of anastomoses, air leaks is checked by inflating the lung after immersing the anastomosis under fluid. In every case, a pedicled flap (intercostal pedicle or pericardial fat pad) is used to encircle the bronchial anastomosis favouring protection and revascularization of the bronchus and to separate it from the PA avoiding broncho-arterial fistulas (Figure 7). A bronchoscopic check of the anastomosis is carried out intraoperatively in order to be able to correct incorrectly placed sutures, stenoses or kinks immediately.
The patient is rapidly extubated, possibly in the operating room. Postoperative management include aggressive physiotherapy for an adequate cleaning of the bronchial secretions and early mobilization, usually on the day after surgery. Mucus retention due to the disturbed mucociliary function can be controlled by intensive preoperative and postoperative physiotherapy and, whenever necessary, by bronchoscopic aspiration. Patient after sleeve resections is treated by low dose steroid inhalation in order to reduce airway secretions and atelectasis, facilitating parenchymal re-expansion, and minimizing the risk of dehiscence and granuloma formation. Before discharge, a bronchoscopy is performed to evaluate the status of the bronchial anastomosis and to identify necroses or smaller fistulae with encapsulated abscesses. Larger bronchopleural fistulae or suture dehiscences may rapidly become clinically noticeable.
Postoperatively the patient received perioperative antithrombotic prophylaxis with low-molecular weight heparin at preventive dose for about 2 weeks.
Comments
Preservation of pulmonary tissue is become a standard practice in lung surgery and lobectomy with bronchial reconstruction is one of the most commonly used technique to spare lung tissue. In fact, sleeve lobectomy is become a useful and accepted surgical procedure among the technical armamentarium of the thoracic surgeon. Different studies on SL showed that the long-term outcomes (survival) were at least equal to those of pneumonectomy but with better functional results. Moreover, the extended use of angioplastic techniques also in the thoracic domain, made possible to perform SL with associated PA reconstruction. These broncho-angioplastic techniques were initially offered to those patients with a respiratory impairment in order to avoid a pneumonectomy which would not tolerate. Initial experiences on this kind of surgical procedures reported an increased rate of morbidity and mortality due to complication of the broncho-vascular anastomosis (broncho-vascular fistula and/or risk of tumor recurrence). In recent years, several published studies on bronco-vascular resection and reconstruction reported that bronchial sleeve resection was associated with both a mortality risk and an incidence of bronchial anastomotic complication lower than that of pneumonectomy, and sometimes comparable to standard lobectomy. Long-term results in terms of survival and disease free interval for this particular type of surgical procedure have documented good outcomes not inferior to those after pneumonectomy. Vogt-Moykopf and colleagues (5,6) reported good long-term results but a high operative mortality (up to 14%). The reported high incidence of complications of the vascular anastomosis (5,6) has led many surgeons to consider angioplastic procedure not safe inducing them to become reluctant to employ this technique. However, recent experiences on broncho-angioplastic procedures showed that they could be performed with good early and late results even after preoperative chemotherapy (5-9). Encouraging results have recently been reported on morbidity and mortality of BAI showing favorable results in terms of operative mortality (3.3%), postoperative complications (32.4%), and 5-year survival rate (38.7%) resulting better than those of pneumonectomy. Our recent results indicate that broncho-vascular resections and reconstruction for treating of central located lung cancer can be performed with a low perioperative risk (7,8).
In conclusion, BAI are safe procedures comparable to bronchial sleeve resections with similar results to those of pneumonectomy. At the light of these results, it seems reasonable to also apply BAI more liberally.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci, Alan D.L. Sihoe and Benedetta Bedetti) for the series “Open Thoracic Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.05.10). The series “Open Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Deslauriers J, Gaulin P, Beaulieu M, et al. Long-term clinical and functional results of sleeve lobectomy for primary lung cancer. J Thorac Cardiovasc Surg 1986;92:871-9. [PubMed]
- Ludwig C, Stoelben E, Olschewski M, et al. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg 2005;79:968-73. [Crossref] [PubMed]
- Takeda S, Maeda H, Koma M, et al. Comparison of surgical results after pneumonectomy and sleeve lobectomy for non-small cell lung cancer: trends over time and 20-year institutional experience. Eur J Cardiothorac Surg 2006;29:276-80. [Crossref] [PubMed]
- Shrager JB, Lambright ES, McGrath CM, et al. Lobectomy with tangential pulmonary artery resection without regard to pulmonary function. Ann Thorac Surg 2000;70:234-9. [Crossref] [PubMed]
- Vogt-Moykopf I, Frits TH, Meyer G, et al. Bronchoplastic and angioplastic operation in bronchial carcinoma: long-term results of e retrospective analysis from 1973 to 1983. Int Surg 1986;71:211-20. [PubMed]
- Vogt-Moykopf I, Fritz TH, Bulzerbruck H, et al. Bronchoplastic and angioplastic operation in bronchial carcinoma. Langenbecks Arch Chir 1987;371:85-101. [Crossref] [PubMed]
- Galetta D, Solli P, Borri A, et al. Bronchovascular reconstruction for lung cancer: does induction chemotherapy influence the outcome? Ann Thorac Surg 2012;94:907-13. [Crossref] [PubMed]
- Galetta D, Borri A, Gasparri R, et al. Surgical Techniques and Long-Term Results of Pulmonary Artery Reconstruction in Patients With Lung Cancer. Ann Thorac Surg 2015;100:1196-202. [Crossref] [PubMed]
- Lausberg HF, Greater TP, Wendler O, et al. Bronchial and bronchovascular sleeve resection for treatment of central lung tumors. Ann Thorac Surg 2000;70:367-71. [Crossref] [PubMed]
Cite this article as: Galetta D, Spaggiari L. Left side sleeves. Shanghai Chest 2017;1:8.