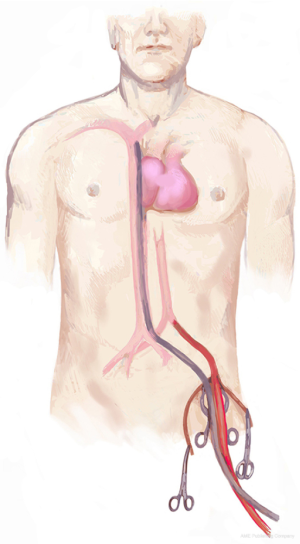
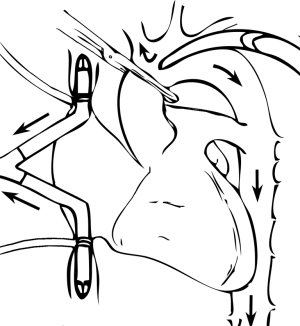
Cardiopulmonary bypass for extended resections
Introduction
Due to their size or location, some thoracic malignancies can involve or displace the great vessels or cardiac chambers. In these circumstances, the use of cardiopulmonary bypass (CPB) may be the only way allowing complete tumor resection, or facilitate the dissection minimising the risk of sudden, non-controllable massive bleeding during surgery. This strategy can be necessary in two clearly differentiated scenarios: the need to resect any infiltrated cardiac structure, usually left atrial wall, or prevention of massive bleeding because of the proximity of tumor to cardiac chambers or great vessels. In this chapter, we are presenting two personal cases and discuss the techniques we recommend in comparable situations. In addition, the procedure must be carefully planned by a multidisciplinary team to choose the more appropriate approach and vascular access which better conform patient characteristics and consider all possible complications. It is also necessary to consider the risks of the procedure and the advantages and inconveniences of non-surgical therapy or palliative alternatives.
Operative techniques
Preventing massive bleeding
Indications and contraindications
Tumours in close contact with vascular mediastinal structures can sometimes be subsidiary of surgery, due to lack of response to chemotherapy, sarcoma pathology type…etc. Some of these situations can make us predict a high risk of bleeding. This can be the case of a tumour which is in close contact or invading vascular mediastinal structures, such as superior vena cava o brachiocephalic trunk, making necessary remove and reconstruct them to achieve complete resection of the malignancy (Figure 1). We could also consider CPB in those cases of highly vascularized tumours in which endovascular embolization is not suitable. In this way CPB help us maintain hemodynamic stability -for instance, with low-flow CPB-, allowing also to cold and rewarm the patient to minimize tissue damage due to hypoperfusion. In those patients with tumours extended to aortic arch or thoracic aorta, CBP allows performing a total circulatory arrest under deep hypothermia. This technique allows us working in a bloodless field without need to control vessels difficult or impossible to access.
However, CPB should be only used in carefully selected patients. It is not a costless technique and can be associated to a high range of complications, most of them due to inflammatory response. In this way, respiratory complications can be up to 49%. Also, cardiac distension and arrhythmia can appear during and after CPB. We should not forget the postoperative risk for haemorrhage, from systemic heparinization, or even central nervous system injury due to inadequate brain protection, mainly in those patients under circulatory arrest.
Patient preparation
Patients whose arterial blood flow is provided and oxygenated by a heart-lung machine are in an abnormal state that affects most of their physiologic processes. This makes necessary to control patient variables and to achieve a great team effort, which includes not only thoracic and cardiac surgeon, but also anaesthesiologists and perfusionists.
Patient must be monitored before surgery begins. It is recommended to place a large-bore—triple lumen- intravenous cannula as well as brachial or radial arterial cannula, and a pulmonary artery flotation catheter. In addition, cerebral oximetry can be considered in some cases. Double-lumen endotracheal tube must also be located. As our patient will be anticoagulated with unfractionated heparin, heparin activity need to be closely monitored. This is commonly done through the activated clotting time (ACT).
Patient position and incision
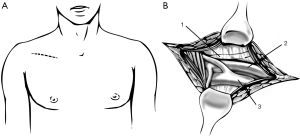
Surgical approach must be adequate to access any structure needing to be resected and allow the vascular access necessary to establish the CPB. Depending on the location and tumour characteristics, incision could be a thoracotomy, median or partial sternotomy, hemiclamshell or clamshell incision. Mid sternotomy provides an excellent access to all cardiac chambers and great vessels, allowing an easy establishment of CPB. It also allows access to both lungs, which is especially easy as they are deflated. But we should remember that hemiclamshell incision (Figure 2) give us excellent access to brachiocephalic vessels, large invasive mediastinal tumours, large central pulmonary lesions and intrapericardial pulmonary vessels.
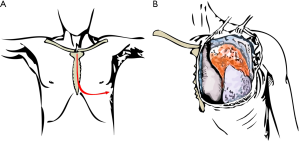
When arterial and venous access—needed to establish CPB—are not possible through the same approach for tumour resection, a peripheral vascular access is necessary. The best option is femoral vessel cannulation (Figure 3). However, if there is significant iliac-femoral disease, axillary artery cannulation is the one recommended (Figure 4).
The patient is placed in supine position for sternotomy as well as for hemiclamshell. For better exposure, we can place a roll or small pillow across both scapulae. Both groins must be prepared and included in surgical field so that femoral vessels can be accessed in case femoral cannulation has been advanced. Pump tubing is passed from the perfusionist to the operating table, completing the CPB circuit.
Detailed surgical technique
The basic premise of our surgery involves a complete en-bloc removal of the tumour. The most critical initial decision is to determine surgical approach. To control most mediastinal structures as well as pulmonary ones, we recommend hemiclamshell incision in these patients.
Patient is placed supine, and an anterior thoracotomy incision in the inframammary crease is first performed up to the sternal midline. The skin incision is then extended vertically upwards to the sternal notch and then for a short distance along the ipsilateral sternocleidomastoid muscle in the neck. The fourth intercostal space is the entrance in most cases, and the internal mammary vessels are divided and oversewn prior to sternal opening. Then sternotomy is performed from above downwards in the midline of the sternum, and curved out to the opened interspace in a J-shaped cut. Bone wax can be spread over the bone marrow, although, in excess, impairs wound healing and increases the risk of wound infection.
While placing the retractor, care should be taken to avoid undue traction on the brachial plexus. When this incision allows to access the ascending aorta and the right atrium, CPB is established through cannulation of these structures. If this is not the case, CPB need to be established through a peripheral cannulation. We must point out that if we expect a difficult access or a high risk of bleeding while thoraco-sternotomy is performed, femoral vessels cannulation should be considered before thoracic incision. CPB is usually not stablished up front but after exploring the chest conveniently.
Femoral vessels cannulation
Before carrying out cannulation patient must be anticoagulated. A single bolus of unfractionated heparin is commonly administered on a weight-based protocol (300–400 units per kilogram), with the goal of achieving an ACT greater than 480 seconds prior to initiation of CPB. Additional heparin bolus is administered periodically during the intervention to maintain desired ACT levels.
The femoral vessels are exposed through an incision in the right triangle of Scarpa, inferior to inguinal ligament. Femoral artery pulse can be helpful to locate vessels placement (1). A curved, oblique or craneo-caudal skin incisions are possibilities to access to femoral vessels. However, a craneo-caudal incision is recommended to improve exposition. As an alternative, J-shaped incision can be done slightly lateral to vessels location to avoid the lympho-ganglionic pad. In any case, it is necessary to perform a meticulous opening and closing technique to avoid a possible lymphorrage.
Common femoral artery and vein are dissected and a vessel-loops are placed around each of them at proximal and distal levels (Figure 5). Whether common femoral artery is short, it may be necessary to control the superficial and femoral profundal arteries with vessel-loops, in order to gain enough access. For femoral artery cannulation, small atraumatic vascular clamps are applied both above and below the intended arteriotomy site. Superficial and profunda artery may either be clamped or snared. A small transverse arteriotomy is made where the arterial wall appears to be relatively normal. A 14–18 F tapered cannula is gently introduced into the arterial lumen and secured in place with a stich joining it to the proximal skin or tying it to the loop already placed around the artery to prevent the cannula to slip out because of perfusion pressure or involuntary movements. The surgeon should always look for a column of pulsating blood in the femoral cannula; in the absence of obvious pulsation, it is very likely that the cannula tip is not in the lumen of the vessel.
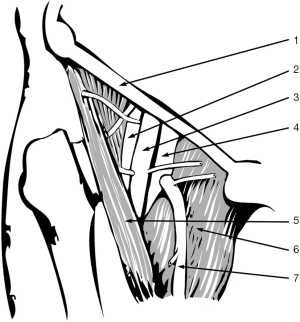
For femoral venous cannulation, a longitudinal venotomy is performed on anterior aspect of common femoral vein. A long 22 o 28 F femoro-atrial cannula with multiple side holes is directly inserted into the vein and advanced, preferably under echocardiographic control, until the distal tip is located into the right atrium. Alternatively, a venous cannula pre-mounted on a tapered dilator core is advanced over a guide wire, previously inserted through a needle puncture in the middle of a 5/0 purse-string polipropilene suture.
In this moment, we can start CPB, and continue with our procedure. We would recommend isolation of the mass from possible adhesions to parietal pleura, resecting it whenever possible, and going on with mediastinal dissection.
Aortic or supra-aortic trunks invasion
In this point, we should inspect the way aorta and the supra-aortic trunk are affected.
Depending on the way aorta is affected, tumour resection can be managed in different forms. Whenever the aortic wall is not infiltrated by malignancy, it is enough to perform a subadventitial dissection. However, when invasion is deeper, partial resection of the aortic wall is mandatory. Although en-bloc excision is recommendable, when aortic wall is invaded or when tumour is of great size, it is safer to leave a tumour piece in contact with the vessel. Its resection will be completed after removing most of the mass. In this situation, and depending on the extension of aortic wall invasion, several options are available. When aortic infiltration area is not extensive and affects a small segment of the aortic circumference, the area to be resected can be excluded through a lateral exclusion with a Satinsky clamp. Aortic wall defect must be repaired with a patch of pericardium or of some synthetic material whose form and size must be properly tailored. Whether this approach is not possible, it is necessary to control the aorta proximally and distally to the lesion. After occluding the aorta with two vascular clamps, the involved segment is completely resected with safety margins, and replaced by a tubular vascular prosthesis of adequate size. For this purpose, a polyester double velour, collagen impregnated, low-porosity prosthesis is recommended. The sutures can then be reinforced with a sealant to ensure hemostasis. Whenever control and occlusion of the aorta are not possible, or are extremely dangerous, the only option is to establish a deep hypothermic circulatory arrest—at an esophageal temperature of 16–18 °C—to perform an open tumour resection and aortic reconstruction in a bloodless field.
When brachiocephalic trunk is the one affected and aorta is not involved, patency of left carotid artery and sufficiency of the Circle of Willis must be evaluated before carrying on its resection. This can be done by checking the effect of temporary occlusion of the innominate artery or right carotid artery in the oxygen saturation of the corresponding cerebral hemisphere, measured by transcranial cerebral oximetry. Once the Circle of Willis competence has been checked, occlusion of the innominate artery proximally and distally to the zone we are going to replace can be performed, even if tubular resection is needed. If we are just going to replace any other supra-aortic trunk, CBP is not compulsory to carry on.
Usually patches and prosthesis used for these purposes are made of woven polyester or PTFE (polytetrafluoroethylene), and we should choose proper size, depending on the artery to be replaced has. Antiagregation is recommended after a patch is placed.
After the procedure, when our mass has been taken out and all these bleeding-risk maneuvering completed, we must stop CBP. Then, venous cannula is withdrawn from the femoral vein and the purse-string suture is tied or venotomy is sutured. Once the arterial cannula has been withdrawn, the arteriotomy is closed with several separated 5-0 polypropylene stitches.
Following discontinuation of CPB and decannulation, heparin is reversed with protamine sulfate in a dose of 1 mg of protamine for every 100 units of heparin. We must remember in this point that protamine administration may be responsible for systemic hypotension or anaphylactic and anaphylactoid reactions, as well as certain grade of pulmonary hypertension.
After positioning drain, meticulous closure involves perfect sternal re-approximation with No 6 stainless steel wires and the use of pericostal sutures for the intercostal space incision, all of which are closed in multiple layers, as well as suturing the thoracotomy as usually done.
Extending lung resection to the atrium
Indications and contraindications
Whether left atrium is involved (Figure 6), partial clamping for resection may cause hemodynamic instability and tumour embolization, depending on tumour size. All this can be avoided with the use of CPB. CPB allows a direct inspection of cardiac chambers, posibilitating to extend the limits of resection to a safer margin.
Patient preparation
Patient preparation is like the previously described. Groins must be also prepared in case any problem appears at the time of central cannulation.
Detailed surgical technique
When tumour is minimally invading the atrium, it could be approached from a thoracotomy, without CPB. In this situation, the interatrial groove must be widely dissected to allow a large Satinsky clamp be placed as centrally on left atrium as possible. In this way, tumor may be completely resected with sufficient safety margins. This maneuver allows a primary closure of the atrial defect with a double 4/0 polypropylene running suture, applied below the clamp
However, when we face a pedunculated lesion or tumor invading the whole atrial wall, CPB is required, to get a complete tumour resection and minimize the risk of embolism, arrhythmias and bleeding. Sternotomy or hemiclamshell are the preferable approaches.
Surgical approach is developed as previously explained and cannulation centrally performed whenever possible. An arterial cannula is placed in distal ascending aorta and single two-staged venous cannula is inserted in right atrium. Two separate venous cannulas—to the superior and inferior vena cava—are necessary when both atrial cavities are to be entered (Figure 7).
When left atrium needs to be opened, that should be done under cardiac arrest. Cardiac arrest is induced by clamping the aorta proximally to arterial cannula and infusing a high-potassium solution (cardioplegia) in the ascending aorta and/or coronary sinus, through previously placed catheters. For that purpose, an aortic root needle is inserted through a 4/0 polipropilene purse-string stitch in mid ascending aorta and a balloon-tiped catheter is inserted in the coronary sinus through the right atrial wall. Cardioplegia is infused for 3 minutes, at a flow of 150 mL/min.m2 for a total dose of 400–750 mL for an average adult (2).
After that, left atrium is opened and tumour is resected, leaving enough free margins. Left atrium closure should be performed with a double-armed 4/0 polipropilene suture with large half-circle needles. When the atrial wall defect is large, it must be reconstructed with a patch of autologous or synthetic pericardium. sutured with the same material.
As soon as we finish atrial reconstruction, we must de-air cardiac chambers to prevent air embolism. We should de-air it through the atrium suture before tying it up. To accomplish it, we recommend the following steps:
- The heart is filled with fluid (blood or electrolyte solution) before closing sutures, to minimize air entrapment.
- Heart must be reperfused and beating.
- Residual air is aspirated from the heart before allowing it to eject.
- Lungs are intermittently ventilated to express air from the pulmonary veins.
- A slotted vent needle is introduced into the aortic opening used to apply cardioplegia, and high suction is applied to it.
An effective way to minimize air embolism is to flood operative field with carbon dioxide during all the procedure. This is achieved by introducing a constant flow of carbon dioxide through a tube anchored to the pericardium above right pulmonary veins.
Heart sometimes starts to beat spontaneously soon after clamp is removed. Nevertheless, in a large majority of cases, a defibrillator can be needed to restore heart rhythm. Asystolia can also be found, and solved using right ventricular epicardial electrodes connected to an external pacemaker.
Apart from atrium excision, to ensure complete tumour resection, sometimes intrapericardial pneumonectomy may be required.
Comments
- It is highly important to identify these patients and schedule step by step this surgery in a multidisciplinary team including cardiothoracic surgeons, anaesthesiologists, and perfusionists.
- En-bloc resection is highly recommended; however, sometimes it is safer to divide the tumour, to dissect mediastinal structures properly.
- When aortic cannulation is needed, we must place purse-string sutures, somewhere with no atherosclerotic or calcified plaques.
- It is also important to choose an appropriate cannula size: if it is too small, it may create a significant gradient in the perfusion pressure while CPB; whereas a large aortic cannula can tear aortic wall or even dissect it.
- Securing femoral cannulas during by-pass is paramount to avoid cannula slippage with perfusion pressure and displacements.
- Despite high range of complications associated to CPB and combined procedures, it is a safe strategy in selected patients (3).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci, Alan D.L. Sihoe and Benedetta Bedetti) for the series “Open Thoracic Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.05.12). The series “Open Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- von Segesser LK. Peripheral cannulation for cardiopulmonary bypass. Multimed Man Cardiothorac Surg 2006;2006(1009):mmcts.2005.001610.
- Konsary S. Cardiac Surgery. Safeguards and pitfalls in Operative Technique. 4th edition. Philadelphia: Wolters Kluwer Lippincott Williams & Wilkins, 2007.
- Muralidaran A, Detterbeck FC, Boffa DJ, et al. Long-term survival after lung resection for non-small cell lung cancer with circulatory bypass: a systematic review. J Thorac Cardiovasc Surg 2011;142:1137-42. [Crossref] [PubMed]
Cite this article as: Fuentes-Gago MG, Arnaiz-Garcia E, Gonzalez-Santos JM, Varela G. Cardiopulmonary bypass for extended resections. Shanghai Chest 2017;1:9.