Open surgery for posterior mediastinal neurogenic tumors
Introduction
Although most of the posterior mediastinal masses are found incidentally in adults, neurogenic tumors are the most common mediastinal tumors in children. The rate of malignancy may be as high as 50% in children, whereas, neurogenic tumors are almost always benign in adults. They are usually symptomatic when they are malignant. It was reported that the tumor extends into the neurogenic canal in 10% of the patients, which are named as “dumbbell tumors” (1). Besides there is an association between von recklinghausen disease and thoracic neurogenic tumors in about 14% of the cases. But this ratio is nearly 50% in adult patients with malignant tumors (2).
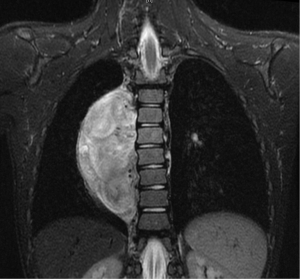
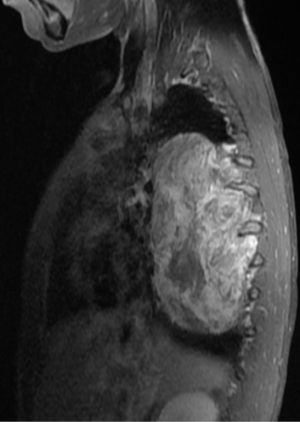
In general a chest computed tomography (CT) is helpful in radiological evaluation. Beside giving information about the presence of a capsule or a cleavage plane, CT scan can also reveal a possible intraspinal extension by demonstrating bony erosion of vertebral pedicles or laminae, or enlargement of an intervertebral foramen. In case of a suspicion about an intraspinal extension of the tumor, magnetic resonance imaging (MRI) is necessary to evaluate the accurate description of the presence and longitudinal extension of the intraspinal component of the tumor (Figures 1-4). Also some particular patients require preoperative angiograph to demonstrate the relation of the tumor with the Adamkiewicz artery.
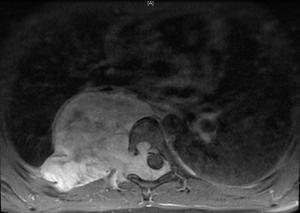
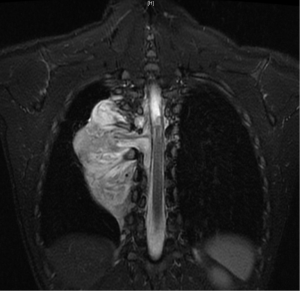
Thurer and Herskowitz specified that “dumbbell tumors” located at lower thorax require more attention during surgery because of the blood supply to the spinal cord (3). During the management of such lesions originating below T6 level, the location of the Adamkiewicz artery must be identified carefully to avoid a possible spinal cold ischemia. This artery supplies the largest part of blood for anterior and posterior spinal arteries at lumbar area. It usually arises between T7 and L4, and mostly between T8-10 intercostal arteries. Spinal angiography may be performed before the surgery. An alternative method is the somatosensory evoked potential measuring during surgery.
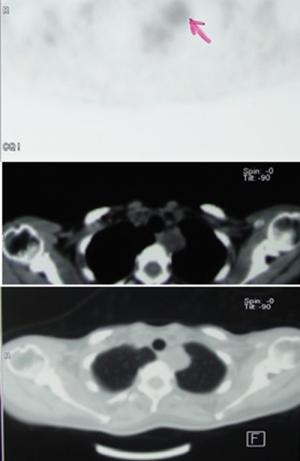
Several authors tried to differentiate schwannomas from sarcomas by evaluating preoperative based on the positron-emission tomography/computed tomography (PET/CT) scans, and proposed a cut off standard uptake value (SUV) max of 4.8 (4). Some studies revealed significantly higher values in patients with benign schwannomas and concluded to still consider the diagnosis of schwannoma despite higher fluorodeoxyglucose (FDG) avidity (Figure 5) (5). Neurofibrosarcomas located at the posterior mediastinum have also demonstrated significant FDG avidity, like sarcomas located elsewhere.
Access to the posterior mediastinum is mostly provided with posterolateral (auscultatory triangle) or anterolateral thoracotomy. Video-assisted thoracoscopic surgery (VATS) has been introduced as a safe and effective method of minimally invasive surgery in the past 15 years. Robotic surgery gained popularity in the past 5 years due to 3 dimensional vision and high technical capabilities of the arms. Open surgery is indicated in large-sized tumors (>6 cm), in the presence of a previous thoracic surgery, or when the tumor is presumed to invade the spinal canal or spinal artery, or is apically located (close to the satellite ganglion, and great vessel). This is because such cases have an increased risk of perioperative complications including avulsion of dumbbell component in the vertebral foramen, bleeding from spinal arteries, cerebrospinal fluid (CSF) leak, injury to the recurrent laryngeal nerve and Horner syndrome. However, in our institution we prefer robotic surgery in every case after excluding a possible intraspinal extension.
Operative techniques
Level of the location, the size of the tumor and its relations to neural foramina and spinal canal are used to determine the incisions and surgical approach.
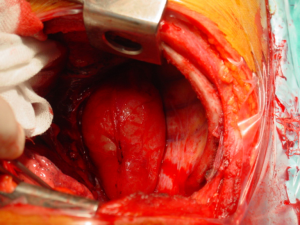
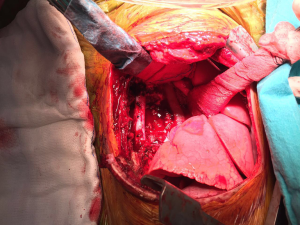
- Mass without obvious clinical or radiological signs of spinal canal involvement: After lung isolation by double lumen endotracheal intubation, the patients is placed in standard posterolateral thoracotomy position. The parietal pleura over the lesion is excised using electrocautery or electrothermal bipolar-activated vessel sealing systems, which can be LigaSure (Valleylab, Boulder, CO) or harmonic scalpel (Johnson & Johnson Medical Products, Ethicon Endosurgey, Cincinnati, OH, USA). During the blunt dissection, care must be taken to both intercostal and vertebral vessels, which should be divided by clipping, if required. The tumor is released from the neural tissue after securing the feeding vessels as demonstrated in the Figure 6. When extrapleural dissection and resection is difficult or impractical technically, intracapsular resection has been proposed for apically located tumors, around the T2 and T3 nerve root closer to larger arteries, veins and stellate ganglion during VATS. In most cases, mass enucleation from subpleural attachment could be performed. The nerve root may be preserved with difficulty in schwannomas, while, in neurofibromas the nerve root is taken en bloc with the tumor. Therefore some authors recommend open surgery instead of intracapsular resection via minimally invasive surgery.
- Mass with clinical or radiological signs of spinal canal involvement.
Two different methods were described for these tumors.
- Akwari defined a combination of posterior and posterolateral approaches. In this method, posterior laminectomy is performed for intraspinal extension of tumor first, then posterolateral thoracotomy is performed for removal of tumor.
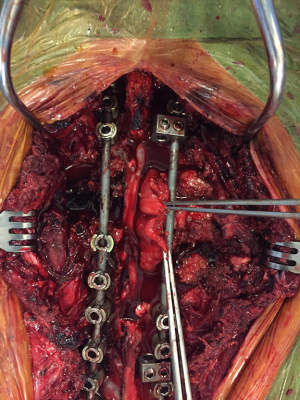
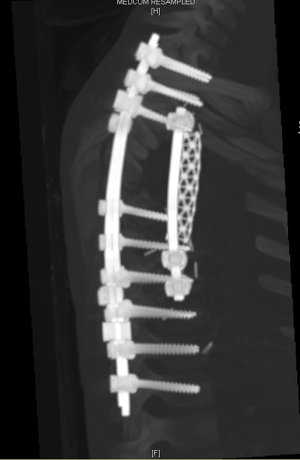
- Spine surgery: Neurosurgeons (spine surgeons) perform posterior laminectomy for “dumbbell tumors”. The patient is positioned prone. Posterior laminectomy is completed at the corresponding levels via a posterior midline incision. Originating nerve roots are divided by ligation in case of a totally extradural tumor (Figure 7). Intradurally extended tumors necessitate further care and experience in spinal surgery. In such case, the dura is opened and the tumor is dissected from the spinal cord. After freeing the intraspinal component of the tumor, it is pushed into the thoracic cavity. The procedure is then completed in posterolateral thoracotomy position. Instrumentation at necessary levels are completed at this stage of surgery (Figure 8).
- Intrathoracic part of the surgery: After skin closure of median posterior incision in prone position, patient is converted to lateral thoracotomy position. We generally prefer a separate thoracotomy incision which approximates the midline of the posterior incision (Figure 9). Sometimes, due to technical difficulties, we combine the incisions to have a wider exposure.
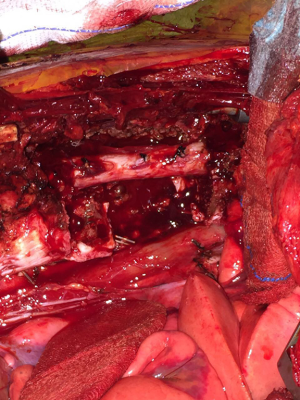
- The dissection of the mass: The thoracotomy is opened in the mid-part of the tumor. By allowing tumor free margins, posterior part of the ribs are divided according to the corresponding planned vertebral resection. The tumor is dissected from the lung (almost always there is not any adhesion), esophagus, aorta, posterior pericardium and main bronchi. A benign tumor never invades the abovementioned structures. Vertebrae are resected after completion of the mobilization of the intrathoracic part of the tumor. Then the prosthetic replacement of the resected vertebrae is completed by spine surgeons (Figure 10).
- Patient is placed in the lateral decubitus position in Grillo’s technique. Rotating ventrally should help accessing to the thorax and spine. In Grillo’s method, a hockey-stick shaped skin incision is performed beginning from vertical mid vertebral area and extending horizontally to the tip of scapula. A flap that consists of skin subcutaneous tissue is lifted over muscle layer, and thoracotomy is performed under this flap tissue. After the exploration and the mobilization of the thoracic portion are completed, resection of intraforaminal extension starts as second part of surgery. Authors suggest that en bloc resection of the tumor with a foraminotomy is possible with thoracotomy incision.
- Akwari defined a combination of posterior and posterolateral approaches. In this method, posterior laminectomy is performed for intraspinal extension of tumor first, then posterolateral thoracotomy is performed for removal of tumor.
However, approaches depend on surgeon’s preferences and experiences. Theoretically one posterior incision is appropriate for small size tumors. When tumors are larger or multiple vertebral foramens are involved Akwari method is preferable according to our experience.
Complications
Postoperative morbidity rate of neurogenic tumor surgery is around 20% to 30%. Pneumonia and atelectasis are among the commonest complications (6). Nowadays, many surgeons prefer to perform these operations using VATS. Although we believe that the conversion to thoracotomy is an oncological must, rather than a complication, 22% to 30% of the patients necessitate conversion because of the intraspinal extension, tumor size, difficult locations of the tumor, or the presence of pleural adhesions. For tumors with intraspinal extension, CSF leak is a frightening complication. Management and identification of these complications needs experience.
Mostly there is no need for a lung resection thus in air-leak and migration of air into intradural space. Increased amount of drainage may be the only symptom. Beta-2 transferrin level in the pleural fluid may help in diagnosis of CSF leakage. The first aim in the treatment is to decrease the intradural pressure by placing a catheter into dura and drainage CSF outside instead of into the thoracic cavity. Revision and closure of dural leak may be alternative if the drainage continues.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci, Alan D.L. Sihoe and Benedetta Bedetti) for the series “Open Thoracic Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.07.01). The series “Open Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Akwari OE, Payne WS, Onofrio BM, et al. Dumbbell neurogenic tumors of the mediastinum. Diagnosis and management. Mayo Clin Proc 1978;53:353-8. [PubMed]
- Inoue M, Mitsudomi T, Osaki T, et al. Malignant transformation of an intrathoracic neurofibroma in von Recklinghausen's disease. Scand Cardiovasc J 1998;32:173-5. [Crossref] [PubMed]
- Thurer RJ, Herskowitz K. Open approaches to posterior mediastinal tumors and the spine. Chest Surg Clin N Am 1996;6:117-38. [PubMed]
- Nose H, Otsuka H, Otomi Y, et al. Correlations between F-18 FDG PET/CT and pathological findings in soft tissue lesions. J Med Invest 2013;60:184-90. [Crossref] [PubMed]
- De Waele M, Carp L, Lauwers P, et al. Paravertebral schwannoma with high uptake of fluorodeoxyglucose on positron emission tomography. Acta Chir Belg 2005;105:537-8. [Crossref] [PubMed]
- Go T, Macchiarini P. Open approaches to posterior mediastinal tumor in adults. Thorac Surg Clin 2010;20:285-95. [Crossref] [PubMed]
Cite this article as: Kaba E, Alomari MR, Toker A. Open surgery for posterior mediastinal neurogenic tumors. Shanghai Chest 2017;1:15.