Tracheal resection and reconstruction
Introduction
Tracheal resection and following reconstruction procedure could be indicated for treating several benign tracheal diseases as well as primary and secondary neoplasm of the trachea. Tracheal stenosis derived from prolonged intubation or previous procedure on trachea (for instance tracheostomy) is largely the most frequent diagnosis leading patients to surgical treatment. The stricture shouldn’t involve a tracheal segment longer than 5 cm or 50% of the entire tracheal length in order to perform a safe tracheal resection and reconstruction.
Indication for surgical treatment and preferred surgical strategy should be established carefully, taking into account the endoscopic assessment of the larynx and airways as well as their radiologic (CT scan) study. The integration of these preoperative studies is able to offer detailed information about the tracheal lesion, which could influence the management of the surgical procedure or, as an alternative, the choice of a more conservative treatment.
It should be underlined that tracheal resection and reconstruction procedures should be carried out in institutions able to offer adequate radiologic support, instruments and expertise for the endoscopic evaluation and treatment of tracheal lesions and the management of potential postoperative complications, intensive postoperative care setting and voice and swallowing evaluations services.
Operative technique
Preparation
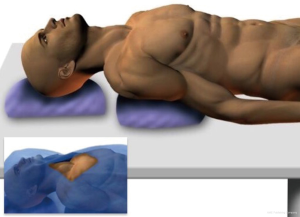
The patient is positioned supine on the operating table.
A surgical cushion is placed horizontally at the level of the scapulae, in order to facilitate the extension of the neck. The head is supported by a circular cushion to prevent hyperextension.
In the right position, chin, neck and sternum should be aligned and horizontally positioned (eventually modifying the surgical bed axis) (Figure 1).
Before starting the surgical procedure is recommended to perform an intraoperative endoscopic evaluation of larynx and airways in order to verify if previous findings indicating the resection are still confirmed. In particular, the following parameters should be carefully assessed: length and diameter of the stenosis, location of the stenosis, mucosal status, stability of the tracheal wall.
Exposition
The sterile surgical field should be represented by an ideal rhombus having the apical vertex in the midpoint of the jaw, the lateral vertex on the midpoint of the claviculae for both sides, and the inferior vertex at the level of the sternal xiphoid process (in the case a sternotomy was needed).
A transverse cervical incision is performed halfway between the inferior border of thyroid cartilage and the sternal notch. The incision should be laterally extended over the sternal portion of the sternocleidomastoid muscle. If a previous scar was present (due to a previous tracheostomy for instance), this could define the level of the incision that should incorporate it.
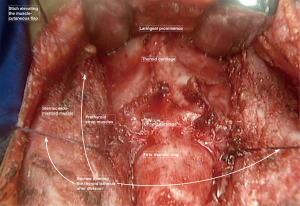
Skin and platysma are dissected until the plane of cervical fascia and pre-thyroid strap muscles. The resulting two muscle-cutaneous flaps are raised in order to expose the deeper surgical field, from the laryngeal prominence to the upper border of the sternal manubrium (Figure 2).
Operation (1,2)
Cervical fascia is opened along the midline and the sternohyoid and sternothyroid muscles are retracted laterally from both sides.
The anterior larynx and tracheal wall are now exposed as well as the isthmus of the thyroid gland, which is divided between sutures (Figure 2).
At this time the tracheal stricture should be identified and its upper and lower borders clearly marked, eventually using the endoscopic view and the transillumination. After that, the pretracheal visceral fascia is opened from the anterior cricoid ring to below the stricture. The opening of this plane should be carefully prolonged until reaching the carina, as it is usually done for the mediastinoscopy procedure. This maneuver permits the anterior mobilization of the tracheal wall (anterior release). At the beginning of the anterior release, it is useful to put at least two (one for each side) lateral stay sutures one or two tracheal ring below the lower border of the stricture in order to traction up the trachea during this procedure. The pretracheal fascia should be open laterally not beyond the tracheoesophageal angles in order to avoid any injury to the recurrent nerves.
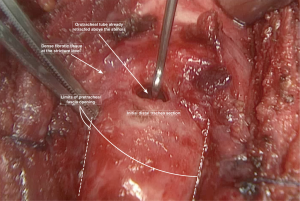
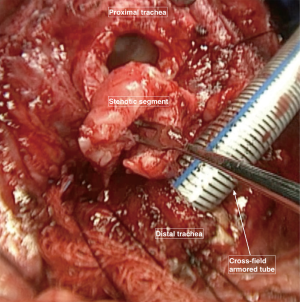
As the stricture’s inferior border is recognized, at this level the diseased trachea is circumferentially mobilized dividing it from the esophagus using a sharp dissection very close to the tracheal wall in order to preserve the nerves (Figure 3). This circular dissection shouldn’t be extended distally toward the healthy trachea, for preserving as much as possible the blood supply of this crucial anastomotic area.
Just below the stricture (eventually through it) the trachea is opened after retracting the orotracheal tube above the stenosis (Figure 3).
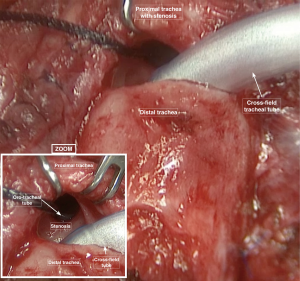
Three other stay suture were applied on both lateral sides and in the middle portion of the distal resected trachea to avoid deformities of the tracheal wall and migration of the distal trachea down to the lower mediastinum. The distal part of the trachea is then intubated using a sterile armored orotracheal tube that is connected to the anesthesia breathing circuit outside the operative field. Once opened and ventilated, the distal trachea could be carefully inspected in order to verify if the divided margin is healthy or if the fibrotic tissue is still involving the margin. In this case, another slice of distal trachea could be resected until obtaining a healthy distal border for the anastomosis (Figure 4) (3).
If necessary the posterior membranous tracheal wall could be divided by blunt dissection from the esophagus till reaching the carina posteriorly. This allows a further mobilization of the distal trachea (posterior release), but obviously this maneuver must spare the tracheoesophageal angle for preserving the recurrent laryngeal nerve and the tracheal blood supply as well (this recommendation is never overemphasized) (4).
At this point, the proximal stenotic tract of the trachea can be pulled up using Allis clamps, in order to divide it from the esophagus and from the surrounding tissues that could be involved in the fibrotic reaction around the diseased trachea. Once the upper border of the stricture is identified and isolated for the whole circumference, the trachea is divided at this level and the specimen removed. Even in this case, the healthiness of the upper tracheal margin should be carefully assessed and possibly a further slice of fibrotic trachea could be removed (Figure 5).
Before performing the tracheal reconstruction, any accidental lesion of the esophagus is carefully evaluated, filling it with a methylene blue solution and looking for potential filtering points.
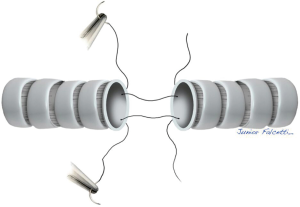
As previously done for the distal trachea, three stay suture should be applied to the proximal trachea. Pulling the upper and lower stay sutures in opposite convergent directions and rotating and pushing the head of the patient towards the sternum, the tracheal superior and inferior borders could be progressively approached each other. In this way the potential tension of the anastomosis is assessed. In case the borders approximate with excessive tension, further release procedures could be performed (as reported within comment section), otherwise the cushion under the scapulae is removed and the reconstruction phase could start.
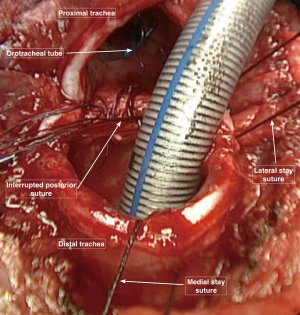
We prefer to perform the end to end anastomosis with interrupted sutures for both anterior and posterior tracheal walls. The stitches are passed entering the lower segment of the trachea (3 to 5 millimeters from the free margin) from outside to inside the lumen and the opposite path from inside to outside is followed for the upper segment (Figure 6). In this way, the knots will be placed outside the tracheal wall for the whole circumference, minimizing the development of granulation tissue within the mucosal surface.
The starting point is represented by the midline of the membranous tracheal wall. Stitches are placed one after the other with a distance of 3–4 millimeters, moving from the middle to the lateral sides, pulling up or laterally the armored ventilating tube in order to facilitate the procedure. Once the suture reaches the stay stitches level, this tube is removed from the surgical field and the orotracheal one is pulled down crossing over the anastomotic line (Figure 7).
It is necessary to pay the utmost attention during this phase to avoid that the tube could pass through any previously settled suture. This could lead to accidentally tie the tube to the anastomosis, with the need of completely reopening the suture at the moment of extubation.
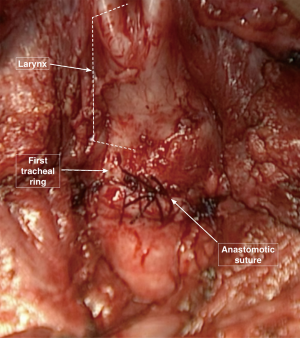
Following the same order of their placement, the posterior stitches are tied with a constant traction on the stay sutures. These stay sutures are tied with the corresponding ones in each tracheal segment, before starting the closure of the anterolateral cartilaginous portion of the trachea (Figure 8).
The complete sealing of the anastomosis is then tested. The endotracheal balloon is deflated and the surgical field is filled with water to cover the suture line. During the ventilation any bubbling site from the anastomosis is detected and the leaking points are carefully closed with an extra suture.
The final anastomosis is buttressed using the strap muscles, that are approximate each other along the midline from both sides with interrupted sutures. If any lesion of the esophagus was previously sutured, usually the left side sternothyroid or the sternohyoid muscles are used to separate the esophagus from the posterior tracheal wall at this level.
Completion
Before proceeding with closure of collar neck incision, it should be evaluated if patient could benefit of positioning a tracheostomy cannula (as later discussed within the caveats paragraph). In this case a short vertical incision (usually involving two rings) could be performed in the tracheal midline preferentially below the anastomosis. A un-cuffed silicon cannula should be placed through the tracheostomy.
Finally, the platysma, the subcutaneous tissues, and the skin are sutured closing the surgical wound. Sometimes, a safety large caliber stitch is applied from the chin to the chest wall at the level of sternal manubrium, in order to prevent the neck extension, which could generate excessive tension on the anastomosis.
Comments
The majority of tracheal resection and reconstruction procedures can be performed using the described standard technique. Nevertheless, during the operation, several problems could arise which need to be managed effectively, keeping in mind that often a second surgical treatment is not possible or burdened by higher risk of morbidity and mortality. In particular, an anastomosis under tension, the contact of the anastomosis with a repaired esophageal lesion or with the innominate artery, and the potential need of a tracheostomy represent crucial situations which should be carefully managed before ending the operation.
Tips
In case an excessive anastomotic tension is verified at the moment of reconstruction, three different release procedures could be performed in order to gain an additional tracheal mobilization of two centimeters with each of them.
The suprathyroid laryngeal release (5) is carried out using the same cervical access already performed. The area between the hyoid bone and the thyroid cartilage is exposed retracting the sternohyoid muscles. The division of thyrohyoid muscle allows exposing the thyrohyoid membrane and the correspondent ligament, which are then resected. This maneuver associated with the resection of the superior thyroid cornua leads to the drop of the larynx.
The suprahyoid laryngeal release (6) is preferably performed through a second surgical incision placed exactly upon the hyoid bone. Once the superior border of this bone is identified, the muscles here inserted (mylohyoid, geniohyoid and genioglossus) are divided from the midline to both lateral sides until reaching the digastric muscle ligaments. The hyoid bone is then resected at the same level just medially to the digastric slings. This procedure exposes the pre-epiglottic space and allows releasing the larynx jointly to the body of the hyoid bone.
The hilar release is rarely performed due to the need of a supplemental thoracotomic access. Usually, the right side is preferred and the pericardium exposed. A longitudinal incision of the pericardium is performed between the phrenic nerve and the anterior border of the main right pulmonary artery and the pulmonary veins. The veins should be encircled by the incision and the pericardium should be divided up to the fibrous pericardial septum (between the pericardium and the inferior vena cava). This maneuver allows mobilizing all the pulmonary hilar structures (right main bronchus, right pulmonary artery and veins) as well as the trachea and moving them cervically.
Caveats
Esophageal perforation
Iatrogenic perforation of the esophagus could be accidentally produced during this kind of surgical procedures, especially while the posterior wall of the tracheal stricture is divided from the esophagus. In order to isolate the stenosed segments of trachea as well as to perform the posterior release as safely as possible, we recommend inserting a nasogastric tube through the esophageal lumen as a landmark. This allows identifying more simply the esophagus by palpation at any time during the surgical procedure. Moreover, before proceeding with the reconstruction phase, we routinely look for any lesion of the esophageal wall filling it with a methylene blue solution. If a perforation is detected, it is usually sealed by interrupted sutures in two layers. The site of repair is isolated by the reconstructed trachea, especially if close to the anastomotic area, using a strap muscle rotated between the posterior tracheal wall and the esophagus.
Risk of tracheoinnominate fistula
In some case, procedures of exposure and mobilization of the trachea and level of anastomosis lead to a close contact between the tracheal suture and the innominate artery. During the early postoperative period, this situation could influence the development of a tracheoinnominate fistula. As a consequence is strongly recommended to separate the artery and the tracheal anastomosis at the end of the reconstruction phase. To this purpose, a strap muscle could be rotated downward in front of the anterior tracheal wall, which will be divided from the artery. It is important to anchor the free margin of the rotated muscle to the tracheal wall distally to the anastomosis in order to completely cover and separate it from other structures.
Need for tracheostomy
At the end of the surgical procedure it could be necessary to perform a temporary tracheostomy, even though the preferred early postoperative management after a tracheal resection and reconstruction is represented by the immediate extubation within the operative room, without placement of any tracheal prosthesis. Nevertheless if any doubt exists about patency of airways or viability of anastomotic tissue or excessive tension at the end of the surgical procedure, positioning an uncuffed tracheostomy cannula seems the safer maneuver. It allows maintaining an effective airway while tissues are recovering from edema and inflammation caused by the surgical procedure and moreover it usually decrease the anastomotic tension facilitating the healing processes. Temporary tracheostomy cannula should be removed after a direct endoscopic assessment of treated airways.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci, Alan D.L. Sihoe and Benedetta Bedetti) for the series “Open Thoracic Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.07.02). The series “Open Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. RMT reports personal fees from Johnson&Johnson, personal fees from Medtronic, personal fees from Astra Zeneca, personal fees from Pfizer, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grillo HC. Tracheal surgery. In: Ravitch M, Steichen F. Editors. Atlas of general thoracic surgery. Philadelphia: WB Saunders, 1987:293-331.
- Grillo HC, Donahue DM, Mathisen DJ, et al. Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg 1995;109:486-92; discussion 492-3. [Crossref] [PubMed]
- Mathisen DJ. Subglottic Tracheal Resection. Oper Tech Thorac Cardiovasc Surg 1998;3:142-53. [Crossref]
- Pearson FG, Cooper JD, Nelems JM, et al. Primary tracheal anastomosis after resection of the cricoid cartilage with preservation of recurrent laryngeal nerves. J Thorac Cardiovasc Surg 1975;70:806-16. [PubMed]
- Dedo HH, Fishman NH. Laryngeal release and sleeve resection for tracheal stenosis. Ann Otol Rhinol Laryngol 1969;78:285-96. [Crossref] [PubMed]
- Montgomery WW. Suprahyoid release for tracheal anastomosis. Arch Otolaryngol 1974;99:255-60. [Crossref] [PubMed]
Cite this article as: Salati M, Terra RM. Tracheal resection and reconstruction. Shanghai Chest 2017;1:16.