
Mediastinal lymph node dissection in open thoracic surgery
Introduction
The importance of mediastinal lymph nodal involvement in lung cancer patients has been identified since 1978, when the first anatomic map for lymph node stations was published by Naruke. In 1997 an updated and detailed map was suggested by Mountain and Dresler and is the one that still remains in use, showing that there is a worldwide consensus on the nomenclature and the fact that they are an important descriptor in the staging process of lung cancer (1-3).
However, such consensus is not evident on the procedure that should be employed during lung resections, in order to obtain the most possible information of lymph node involvement and provide an accurate, final, postoperative, pathological N stage for non-small cell lung cancer. There is an evident long term dispute in the literature between the supporters of complete lymphadenectomy versus sampling (4,5). Equally, discussions remain whether a surgeon should perform lymphadenectomy in every mediastinal station or should follow a lobe-depended approach, in order to decrease the internal operative trauma (6). At the moment, the gold standard for the treatment of non-small cell lung cancer is lung resection combined with complete lymph node dissection of at least 3 mediastinal (N2) stations (7,8).
Operative technique
The traditional thoracotomy provides excellent access for an extensive lymph node dissection. The timing is a subject of discussion but we advocate lymphadenectomy prior to lung resection for the following reasons:
- Suspected lymph nodes can be subjected to intra operative frozen section and alter the surgical management in certain Institutions (9);
- Prior lymphadenectomy offers a superb dissection of the hilum with identification and separation of all hilar structures;
- Provision of a ‘clean’ operating field for safe ligation and transection of hilar structures;
- Safeguards margins and eliminates disputes between surgeons and pathologists;
- Allows en bloc, clean resection of nodes and fat pads providing the best specimens for pathological examination.
Lymphadenectomy
General aspects
The preparation of the patient, incision and access to hilum and mediastinum have already been described in other chapters.
For the sake of completeness, the following safety features apply to each patient undergoing lymphadenectomy:
- Appropriate exposure with good lung isolation;
- Prior complete adhesiolysis with exposure of hilum and mediastinum;
- A ‘dry’ field with good prior hemostasis as blood pools in the most dependent areas which are peri hilar and mediastinal;
- A sound knowledge of anatomy of mediastinum.
Technique description
Station 2
This station is also called “upper paratracheal” and it is usually dissected at the same time with Station 4 or by extending the dissection planes from Station 4 superiorly (Figure 1). For right sided resections the access is easier following the groove between the trachea and the superior vena cava (SVC), although on the left side the aortic arch obscures the surgeon’s view and special maneuvers need to be employed.
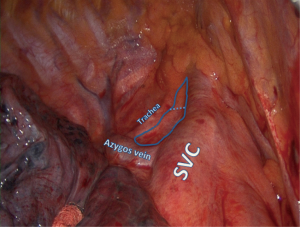
The assistant retracts the lung inferiorly. The pleura is incised lateral to the phrenic nerve and at the superior border of the azygos vein or aortic arch for right or left approach respectively. The fat tissue is grasped elegantly with fenestrated forceps or plain forceps and is dissected bluntly from the tracheal wall and surrounding structures. Diathermy is discouraged in this area as right sided dissection comes close to the right recurrent laryngeal nerve. Bleeding is avoided by clipping small arterial branches to the lymph nodal pad and avoiding injury to small draining veins that connect directly to the SVC on the right side. Small bleeds should be treated with simple gauze packing while other jobs are done in the chest. In the majority of cases no further action is required and hemostatic agents are available but rarely necessary.
Station 4
This station is also called “lower paratracheal”. For a right approach the access is easy, but on the left the exposure is obstructed completely by the aortic arch and dissection on the left side is not routinely performed.
The lung is retracted inferiorly. The pleura is incised around the anterior and superior hilum, so that the azygous vein is separated from the pulmonary artery, lying inferiorly. The lower margin of Station 4 is identified below the proximal segment of the azygous vein resting posterolaterally to the SVC. The lymph nodes are dissected bluntly with a peanut or the suction catheter away from the azygous vein and SVC and away from the right main pulmonary artery (Figure 2).
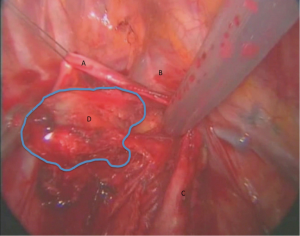
The detachment from the artery may be facilitated by opening the arterial sheath, so at the same time the surgeon can assess any possible involvement of the pulmonary artery. The use of diathermy is not encouraged in this area, because of close proximity to major vascular structures. However, an energy device can facilitate the dissection without blood loss, maintaining clear dissection planes. This part of the station can be either removed at this stage or left aside and retracted from above the azygous vein for the next stage of dissection.
Hemostasis follows the same principles as described for station 2. Quite often these 2 stations are taken en bloc and an arbitrary division is performed by scissors. The specimens are placed in separate pots to be examined by a pathologist.
We generally advocate the use of clips and high energy devices to avoid injury to major vessels and the right recurrent nerve. Additionally, a clean dissection reduces the risk of an ‘annoying’ chyle leak, experienced when aberrant thoracic duct branches are disrupted but not ligated.
Station 5
We find this station only in the left chest cavity. It is also called “subaortic” and is located between the aortic arch and the left main pulmonary artery, just laterally to the ligamentum arteriosum (aortopulmonary window).
The assistant retracts the lung inferiorly while the surgeon opens the pleural reflection at the superior border of the hilum. It is advisable to commence dissection by staying in close proximity of the left main pulmonary artery and identify the vagus nerve. The fat pad is gradually detached, vagal branches are clipped and no diathermy is used to avoid injury to the left recurrent laryngeal nerve. The dissection always keeps the roof of the vagus nerve as a pivot point and ascends as it comes to close proximity of the aortic arch. Quite often a ‘button’ of parietal pleura is sacrificed in order to perform a complete undisrupted dissection of the nodal fat pad.
Any minor bleeding is attended by gauze packing.
Station 6
It is also called “para-aortic” and is located adjacent to the lateral wall of the ascending aorta and aortic arch and its dissection is usually performed at the same time with the Station 5.
The dissection is a true continuation of the Station 5 lymph nodal pad. Injury to the left vagus should be avoided as it lies on the aortic arch and anterior dissection should protect the left phrenic nerve as well.
Station 7
This station is also called “subcarinal”.
Station 7 is deeply embedded in the posterior mediastinum and access is impossible or challenging without prior appropriate homework from the surgeon.
Regardless of side of operation the pleural reflection on the posterior hilum needs to be dissected. The vagus nerve is identified and all hilar branches are individually identified and ligated. This maneuver offers two advantages:
- Prevents any bleeding from bronchial tributaries;
- Allows the ligatures to retract the vagus with the posterior mediastinum offering a good exposure.
On the left side, the proximal descending aorta reduces further the operating field and at times it needs to be retracted.
The main bronchus remains the pivot point on both sides. As soon as the nodes are identified the dissection concentrates on the inferior aspect of the main bronchus. The retraction of the nodes should be gentle and they should not be disrupted. Anteriorly the nodal pad is dissected from the posterior pericardium. There are generally no vessels of concern in that territory.
At the posterior aspect, the nodal pad needs to be dissected from the esophagus (Figure 3). We advocate the use of clips and no diathermy as the esophagus is an elegant structure and thermal injuries should be avoided with the generous use of diathermy. If in doubt, the surgeon should ask the anesthetist to introduce a large bore nasogastric tube or an esophageal bougie. Such maneuver will stent and state better the esophagus in the operating field. Identification is then easier in case of significant fibrosis and difficulty in identifying the correct dissection planes.
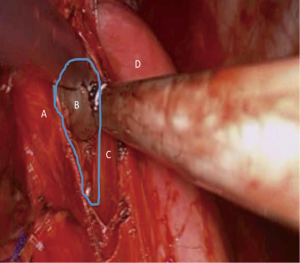
As soon as the true subcarinal area is reached care should be taken to clip the subcarinal bronchial vessels. For dissections performed on the background of inflammatory disease or bulky lymphadenopathy such vessels can be large and significant bleed might be encountered which is difficult to control at times due to the proximity of the vessels to the carina.
The reader should remember that the subcarinal dissection exposes the membranous part of the airways. This is subject to injuries as pressure is also applied endobronchially with the double lumen tube.
In case of a wet field, gauze packing is the answer again. The worst mistake is to control bleeding in a confined space with limited dissection and visibility.
In extreme circumstances, a period of apnoea with the bronchial cuff deflated might offer the extra volume required in the operating field to take care of the bleeding.
The subcarinal space should be routinely packed for a few minutes while other jobs are done. The use of haemostatic agents is rarely necessary.
Station 8
This station is also called “paraoesophageal” and is including the lymph nodes that lie adjacent to the esophagus in each side, between the level of the bronchus and the diaphragm.
This is again a continuation of the previously described dissection of the posterior mediastinum and subcarinal nodes. This time the dissection identifies the paraoesophageal nodal pad just inferior to the subcarinal nodes and simply follows the esophagus until the pad is lifted off with gentle dissection and use if clips.
Again, the protection of the esophagus is of paramount importance. Additionally, the dissection should remain on the boundaries of the nodal pad to avoid injury to the thoracic duct leading to a chyle leak. For this reason, the separation of this nodal station from the mediastinum should ideally be performed mainly with blunt dissection and use of clips or ligation.
Station 9
This station is also called “inferior pulmonary ligament node”, as it is lying inside the attachment of the pulmonary ligament to the mediastinum, between the inferior pulmonary vein (IPV) and the diaphragm on each side.
The lung is retracted superiorly and the ligament is transected initially with diathermy or a high energy device. Blunt dissection will then reveal the nodes within the pleural reflection and these should be dissected with the fat pad and part of the inferior pulmonary ligament until the floor of the IPV is identified (Figure 4).
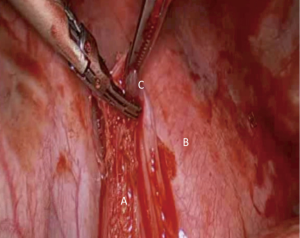
This area has limited blood supply and bleeds are rarely encountered except the posterior attachments with the mediastinum and the close proximity to the IPV. The reader should remember that clips at the floor of the IPV should be avoided as subsequent stapling of the vein during lung resection can lead to stapler failure and catastrophic bleed.
General comments and advice
A meticulous lymph node dissection does not cure cancer but streamlines cancer treatment by offering accurate staging. Evidence shows that there is significant upstaging in patients who underwent total lymphadenectomy in comparison to those who had lymph node sampling. Hence, these patients can be offered adjuvant treatment with potential improvement in their long-term prognosis. Taking this into consideration, surgeons should be capable of offering their patients a safe, complete lymph node dissection without compromising patient safety.
The reader should keep in mind the following tips:
- In any nodal station, the surgeon should follow the landmarks surrounding the nodes, to facilitate a dry dissection;
- The technique of ligation instead of diathermy dissection keeps the field dry, protects surrounding tissues from potential thermal injuries and reduces the incidence of postoperative chyle leak from lymphatic vessels;
- The recurrent laryngeal nerve should be protected in lymphadenectomy of Stations 2 in the right side and 5 in the left side, especially in cases that there are no preoperative findings suggestive of involvement in these stations, so that a potential injury can justify the final disability;
- In patients with mediastinal lymphadenopathy and neoadjuvant treatment, the operating field might be hostile. The dissection should follow natural reflections and the soft flat structures, i.e., esophagus will need to be identified with special maneuvers to avoid injury (use of bougie or large nasogastric tube);
- Metal clips are a good choice for ligating small feeding vessels at surgery. They assist in an accurate dissection but are future markers if adjuvant radiotherapy is required.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Harisinghani MG. Atlas of Lymph Node Anatomy. New York: Springer, 2013:31-56.
- Watanabe S. Lymph node dissection for lung cancer: past, present, and future. Gen Thorac Cardiovasc Surg 2014;62:407-14. [Crossref] [PubMed]
- El-Sherief AH, Lau CT, Wu CC, et al. International association for the study of lung cancer (IASLC) lymph node map: radiologic review with CT illustration. Radiographics 2014;34:1680-91. [Crossref] [PubMed]
- Dong S, Du J, Li W, et al. Systematic mediastinal lymphadenectomy or mediastinal lymph node sampling in patients with pathological stage I NSCLC: a meta-analysis. World J Surg 2015;39:410-6. [Crossref] [PubMed]
- Doddoli C, Aragon A, Barlesi F, et al. Does the extent of lymph node dissection influence outcome in patients with stage I non-small-cell lung cancer? Eur J Cardiothorac Surg 2005;27:680-5. [Crossref] [PubMed]
- Adachi H, Sakamaki K, Nishii T, et al. Lobe-Specific Lymph Node Dissection as a Standard Procedure in Surgery for Non-Small Cell Lung Cancer: A Propensity Score Matching Study. J Thorac Oncol 2017;12:85-93. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Whitson BA, Groth SS, Maddaus MA. Surgical assessment and intraoperative management of mediastinal lymph nodes in non-small cell lung cancer. Ann Thorac Surg 2007;84:1059-65. [Crossref] [PubMed]
- Li W, Yang XN, Liao RQ, et al. Intraoperative frozen sections of the regional lymph nodes contribute to surgical decision-making in non-small cell lung cancer patients. J Thorac Dis 2016;8:1974-80. [Crossref] [PubMed]
Cite this article as: Kostoulas N, Papagiannopoulos K. Mediastinal lymph node dissection in open thoracic surgery. Shanghai Chest 2017;1:22.


