
Open surgical approach and technique in left pneumonectomy
Operative technique
Preparation
All patients should have a rigid or flexible bronchoscopy. Left lung isolation should be performed with a right-sided double lumen tube as the left main bronchus must be free of endoluminal objects.
Following confirmation of isolation, the patient is placed in the right lateral position. The right knee is flexed at 90 degrees, the left lower limb remains straight and a pillow is cushioned between them. Both upper limps are flexed at ‘praying’ position to allow exposure of the left axilla. There is no need to place the left arm on a frame. Flexing the operating table at the level of the xiphoid process splays the intercostal spaces (Figure 1). The patient is secured by using a special ‘bean’ bag and a wide strap across the pelvis.
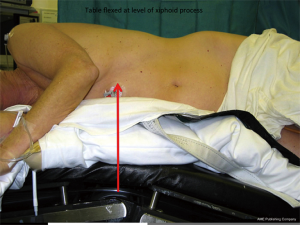
The patient is prepped from the left shoulder to the pelvis and from the spine to the sternum, including the left axilla. In the same fashion drapes are placed around the above mentioned anatomical boundaries.
Incision
Access to the chest cavity can achieved either by anterior or posterolateral thoracotomy.
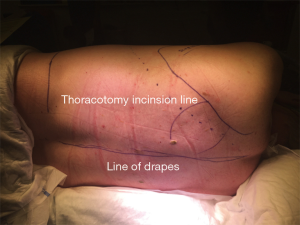
In our Institution, the posterolateral approach is recommended as rib approximation is easier and minimum disruption of muscle fibers is required through the posterior auscultatory triangle (Figure 2).
The presence of adhesions is not a contraindication to surgery but requires extra skills with attention to:
- Identification of appropriate planes;
- Dry division of adhesions with haemostasis;
- Avoidance of visceral pleura disruption and;
- Complete adhesiolysis with mobilization to the whole lung and access to all hilar structures.
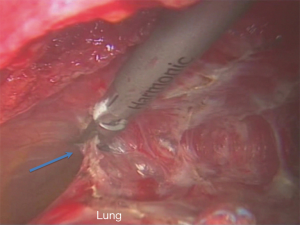
When such maneuvers are not possible an extra pleural dissection is the way forward. The surgeon should remain confident that the provision of correct planes does not compromise an oncological operation and cancer margins are not compromised (Figure 3).
Operation
A thorough inspection of the pleural cavity is important to exclude pleural deposits in pneumonectomies for lung cancer. Furthermore, any unsuspected pleural effusion should be sent for cytology in cancer operations (1,2).
Hilar dissection (3)
This step of the operation has several benefits including:
- Assessment of each hilar structure;
- Recognition and preservation of mediastinal structures;
- Assessment of resectability in cancer surgery;
- Full lymphadenectomy or lymph nodal sampling if required.
The assistant retracts the left lung medially and the hilum is dissected at the level of the left pulmonary artery (PA). Following disruption of the parietal pleura all fatty and nodal tissue at the aortopulmonary window is resected isolating small vessels and branches of the vagus nerve with clips or ligatures. Diathermy is discouraged at this territory due to the close proximity of the left recurrent laryngeal nerve (3,4).
The main trunk of the left pulmonary artery is dissected with blunt dissection. Care is taken to avoid injury to the vessel wall using gentle finger rolling dissection and/or blunt dissection with a ‘peanut’. A clamp is positioned around the vessel only when circumferential dissection has been achieved and a vascular tape is applied and allowed to rest in the thoracic cavity.
The dissection continues posteriorly and inferiorly in the groove between the lung hilum and the esophagus. At this point, bronchial vessels might be encountered as well as bigger branches of the left vagus nerve. We prefer individual ligation with retraction of ligatures which allow suspension of the posterior mediastinum from the lung hilum. At this point, all left 10 lymph nodes are dissected from the groove between the left pulmonary artery and the bronchus and careful haemostasis is achieved.
Further dissection inferiorly exposes the left inferior pulmonary vein. The inferior pulmonary ligament needs to be divided until the rest of the vein is exposed circumferentially and all left 9 lymph nodes can now be exposed and harvested (Figure 4). It is now easy to tease off the rest of the circumference of the vein with finger dissection, apply a clamp (Figure 5) and circumvent the vein with a vascular tape. The tape is again allowed to rest in the thoracic cavity.
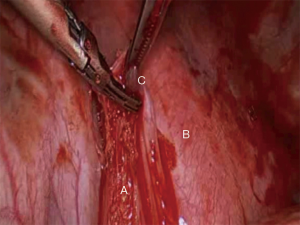
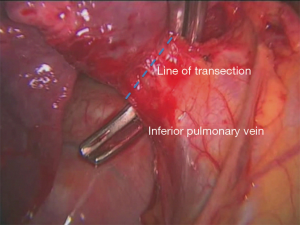
The hilar dissection is guided now anteriorly by simply retracting the lung laterally. The phrenic nerve should be identified, especially in obese patients in whom it might rest within a generous pericardial fat. The nerve should be freed from the hilum with blunt dissection. No diathermy should be employed at this stage of the dissection (5,6).
The circumferential dissection will now allow exposure of the last hilar structure, the superior pulmonary vein. Quite often this proves to be multi-segmental and wide and the dissection should be facilitated by the index finger and thumb taking the previously dissected pulmonary artery as a pivot point at the most superior aspect of the vein. In a similar fashion the vein can be encircled with a vascular tape.
The last hilar structure is the left main bronchus, but accessibility at this point is extremely difficult as it is surrounded by all vascular hilar structures.
There are various techniques and instruments that may be used for dividing the pulmonary vessels. The easiest and most efficient way nowadays is by dividing the vessels with readily available vascular staplers. They can be easily applied in the operating field through the thoracotomy incision and slide behind each vessel by following the previously applied vascular tape or sling.
In case that a surgeon wishes to perform a very small thoracotomy the staplers can be manipulated through an anterior small incision which will serve later as the chest tube port (7).
We prefer to transect the pulmonary artery in order to avoid lung congestion if veins are sacrificed first (8). Using the vascular tape, the PA is gently retracted and the anvil of the stapler is slid underneath the artery lumen in a medial to lateral fashion. In those who prefer a small access thoracotomy, direct vision might be difficult. In such cases the right index finger of the surgeon might be placed around the PA and the anvil positioned against the tip of the index finger which serves as a guide for safe passage of the stapler jaw under the PA. Care should be exercised to isolate the full diameter of the PA before applying the stapler. Vascular clamps should be at close proximity in case of hemorrhage.
The inferior pulmonary vein can now be transected with the stapler sliding from the pericardial aspect towards the posterior mediastinum. Care should be exercised to remove the vascular tapes before firing the staplers to avoid these being trapped at the proximal stump of the vein.
The superior pulmonary vein can now be transected in a similar fashion. The lung is now much easier to manipulate as it is anchored only by the main bronchus.
In practices were staplers might not be available or financially affordable classic techniques of suturing or ligation might be employed. In general terms, the pulmonary artery can be isolated with 2 vascular clamps, neatly divided and then over sawn with non-absorbable monofilament suture with an atraumatic needle such as prolene 4/0. Veins can be taken care in a similar fashion. We would not advocate the use of single ligatures as these might slip and end up in a catastrophic bleed. Our experience suggests that veins are more susceptible to such accidents as they often have a pericardial reflection rendering safe ligation a risk.
The distal stumps of the vessels can be ligated with comfort but we would advocate transfixion as distal bleeding exposes a surgeon to unnecessary discomfort, disrupts the flow of the operation and leads to a ‘wet’ and hostile operating field.
As described earlier, the division of the bronchus can be employed either by the use of a stapler or sutures.
Before transecting the bronchus, care should be taken to remove all subcarinal nodes if surgery is performed for cancer. The bronchus should be cleaned from surrounding fat and the dissection should ensure that the subcarinal region is reached. This maneuver will guide the position of the stapler and prevent a long bronchial stump be left behind. The stapler can be routinely applied in an inferior to superior direction with the lung retracted posteriorly. Quite often the stapler can compress the aortic arch to secure a short remaining bronchial stump.
At stapling, the lung is retracted and the stapler is applied to the bronchus by collapsing the membranous part against the anterior wall. During stapling the tension on the lung should be released to avoid rupture of the bronchus.
If sutures are to be applied the bronchus should not be transected to avoid soiling the pleural cavity with respiratory flora. Specially designed curved bronchial clamps are available and should be applied close to the carina. The bronchus is transected allowing the lung to be extirpated from the pleural space.
With the bronchial clamp in situ, the bronchus is sutured with a double horizontal mattress technique with mono or poly filament suture with a cutting needle (9). There is enough evidence in the literature showing that monofilament or poly filament sutures can be successfully employed providing that:
- Good bronchial wall approximation is achieved (10);
- The bronchial tissue is healthy and no suture overcrowding is employed;
- The bronchial stump is short.
It is not necessary to cover the left bronchial stump routinely as it is naturally covered by mediastinal structures unless (11,12):
- The bronchial tissue quality is questionable;
- The patient has received preoperative chemotherapy and/or radiotherapy;
- The patient will require adjuvant chemotherapy and/or radiotherapy;
- The operation has been performed in an infected or inflamed pleural cavity;
- The patient’s nutritional status is poor.
Completion
Following or completing the lymphadenectomy the pleural cavity is washed with water (13,14). The bronchial stump can be checked for possible air leaks. If such is identified care should be taken to isolate it with fine sutures.
There has been significant discussion regarding the use of chest tubes following pneumonectomies. Either practice has proved to be safe and effective as long as the appropriate protocols are followed.
In our Institution, a single chest drain is left in the chest until the first postoperative day (POD 1). Our protocol consists of:
- Clamping the drain in theatre after the patient has been turned in supine position;
- Open the drain every hour to check for bleeding;
- Keep it clamped as soon as no drainage is identified;
- Remove the drain the following morning (POD 1).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci, Alan D.L. Sihoe and Benedetta Bedetti) for the series “Open Thoracic Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.08.10). The series “Open Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saso S, Rao C, Ashrafian H, et al. Positive pre-resection pleural lavage cytology is associated with increased risk of lung cancer recurrence in patients undergoing surgical resection: a meta-analysis of 4450 patients. Thorax 2012;67:526-32. [Crossref] [PubMed]
- Shoji F, Yamazaki K, Kouso H, et al. The Impact of Pleural Lavage Cytology Both Before and After Lung Resection on Recurrence of Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:2141-6. [Crossref] [PubMed]
- Nohl-Oser HC, Nissen R, Schreiber HW. Surgery of the lung. Stuttgart, New York: Thieme-Stratton, 1981:79-90.
- Filaire M, Mom T, Laurent S, et al. Vocal cord dysfunction after left lung resection for cancer. Eur J Cardiothorac Surg 2001;20:705-11. [Crossref] [PubMed]
- Burns J, Dunning J. Is the preservation of the phrenic nerve important after pneumonectomy? Interact Cardiovasc Thorac Surg 2011;12:47-50. [Crossref] [PubMed]
- Kocher GJ, Mauss K, Carboni GL, et al. Effect of phrenic nerve palsy on early postoperative lung function after pneumonectomy: a prospective study. Ann Thorac Surg 2013;96:2015-20. [Crossref] [PubMed]
- Ugalde P, Miro S, Provencher S, et al. Ipsilateral diaphragmatic motion and lung function in long-term pneumonectomy patients. Ann Thorac Surg 2008;86:1745-51; discussion 1751-2.
- Szwerc MF, Landreneau RJ, Santos RS, et al. Minithoracotomy combined with mechanically stapled bronchial and vascular ligation for anatomical lung resection. Ann Thorac Surg 2004;77:1904-9; discussion 1909-10.
- Refaely Y, Sadetzki S, Chetrit A, et al. The sequence of vessel interruption during lobectomy for non-small cell lung cancer: is it indeed important? J Thorac Cardiovasc Surg 2003;125:1313-20. [Crossref] [PubMed]
- Scott RN, Faraci RP, Hough A, et al. Brochial stump closure techniques following pneumonectomy: A serial comparative study. Ann Surg 1976;184:205-11. [Crossref] [PubMed]
- Kakadellis J, Karfis EA. The posterior membranous flap technique for bronchial closure after pneumonectomy. Interact Cardiovasc Thorac Surg 2008;7:638-41. [Crossref] [PubMed]
- Panagopoulos ND, Apostolakis E, Koletsis E, et al. Low incidence of bronchopleural fistula after pneumonectomy for lung cancer. Interact Cardiovasc Thorac Surg 2009;9:571-5. [Crossref] [PubMed]
- Lardinois D, Horsch A, Krueger T, et al. Mediastinal reinforcement after induction therapy and pneumonectomy: comparison of intercostal muscle versus diaphragm flaps. Eur J Cardiothorac Surg 2002;21:74-8. [Crossref] [PubMed]
- Tsakok T, Tsakok M, Damji C, et al. Washout after lobectomy: is water more effective than normal saline in preventing local recurrence? Interact Cardiovasc Thorac Surg 2012;14:200-4. [Crossref] [PubMed]
Cite this article as: Kostoulas N, Papagiannopoulos K. Open surgical approach and technique in left pneumonectomy. Shanghai Chest 2017;1:23.


