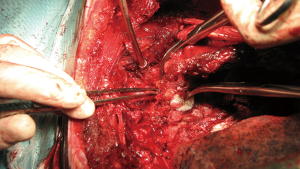
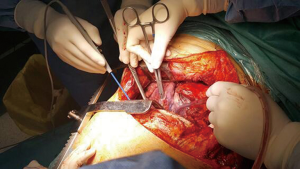
Posterior thoracic approach for Pancoast tumour resection
Introduction
Pancoast tumour is an uncommon lung cancer presentation, it represents only 3–5% of all lung cancer. Located at the superior pulmonary sulcus with direct spread to the para-apical structures makes its clinical presentation atypical and usually delays the diagnosis and the further treatment. Clinical presentation usually consists in Pancoast’s Syndrome. Radicular arm affection caused by brachial plexus infiltration, shoulder pain because of rib tumour invasions and Horner’s syndrome (miosis, ptosis and anhidrosis) in relation to sympathetic nerve involvement (1-3).
Small apical tumours could remain hide behind the clavicle and first ribs and be missed on plain chest X-rays in early stages. In consequence when clinical presentation suggests the presence of this pathology CT scan becomes the gold standard for diagnosis and staging as well as to define the size of the process and reveal bone, spinal, mediastinal or brachial plexus invasion, detect peripheral or satellite lesions and assess the presence of enlarged lymph nodes. Magnetic resonance imaging proportionates a more accurate local extension preoperative evaluation. In our opinion, CT guided biopsy for histological diagnostic is mandatory—especially in locally extended tumours—previous to continue the operability assessment and therapy strategy planning. PET-CT is useful for preoperative detection of lymph nodes involvement and occult metastatic disease. Because of high rate of false negatives reported on PET-CT, cervical mediastinoscopy is still considered mandatory (2).
Non-hiliar lymph node metastases (N2 or N3) are associated with lower 5-year survival. However, ipsilateral supraclavicular lymph node involvement (N3) demands a special mention. As a result of better survival in these cases compared to N2 affection, supraclavicular lymph node involvement may be considered as a first lymphatic drainage (N1) and do not contraindicate surgery (4).
Globally less than 50% of patients with Pancoast tumours are considered resectable at diagnosis. Induction chemo-radiotherapy has been proposed with the intention to increase resectability options, particularly in doubtful cases (1,4). Surgical resection is most commonly accomplished with either posterior or anterior approach. Posterior thoracotomy described by Shaw-Paulson as a classical postero-lateral thoracotomy extended around the scapula to the base of the neck, has certain advantages over the anterior approach. It is ideal for posterior tumours, particularly those that invade the vertebral bodies and the brachial plexus. Also if invasion of the subclavian artery is found, this approach can still be used (1,4).
Surgical technique
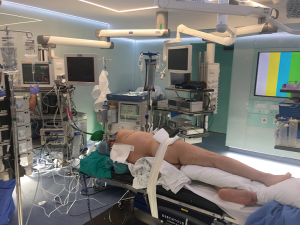
Under general anesthesia a double lumen tube is placed—preferably a contralateral specific bronchus tube. Arterial line for gas analyses and blood pressure continuous monitoring is place at contralateral arm. A deep venous line—subclavian or jugular—is placed at the same side of the tumor. Epidural catheter is mandatory for analgesia and postoperative pain management. It is preferable to place it before surgery to start analgesia as soon as possible to reduce chronic pain. Thereafter the patient is positioned and secured in lateral decubitus. Inferior leg is flexed and superior stays extended placing a pillow between both legs. The ipsilateral arm is fixed at double 90º positions using an arm holder or a pillow. Table is broken at the level of xifoid to widen the intercostal space. Especial care must be taken in protecting the brachial plexus and cubital nerve as well as in cushion the decubitus areas (Figure 1).
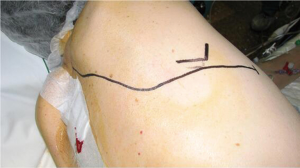
Sterile draping is done exposing spine and nipple to be used as antero-posterior landmarks. Scapula and costal borders are the supero-basal landmarks. Sterile skin adhesive protection is placed to prevent cutaneous contamination (Figure 2). After security checklist is done, a postero-lateral thoracotomy is undergone extending it following the interescapulo-vertebral space (Figure 3).
First, Latissimus Dorsi is exposed and completely divided. Both sides of the muscles are desinserted for a better exposure and to facilitate closure at the end—this is easily done everting the muscle. Second, the Serratus muscle is preserved and the inter-scapulo-romboid fascia is opened. Posteriorly the trapezium and rhomboids muscles are divided to expose de paravertebral muscles and costovertebral junction. Finally, 4th intercostal muscle is desinserted from the 5th rib to enter to pleural cavity.
Once the pleural cavity is opened a small retractor is placed anteriorly to spread the ribs. For easier exposure and to avoid an accidental rib fracture we recommend an internal further division of the intercostal space. Some times if exposition is not good enough posterior costectomy of the 6th rib could be necessary.
Before chest wall resection the pleural cavity has to be thoroughly explored. Attachments must be released and the tumor borders precisely identified to ensure that resectability is still possible. In addition exploration for intracavitary unexpected metastases is important before start resection.
Recently, some groups advocated for VATS approach of chest wall-involving tumors. In those situations the lobectomy is performed prior to the wall resection. However, in our opinion, in posterior tumors with required costal desarticulation VATS surgery is not recommendable. In our experience, good short-term pain management and survival results were obtained using an open approach.
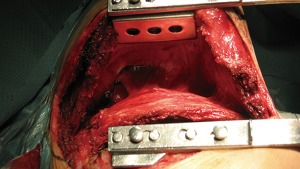
Before resection, complete exposition of the upper thoracic inlet and costo-vertebral junction is mandatory. Rib spreader is used to elevate the scapula placing one blade to the 5th rib border and the other one on the tip of the scapula—Finochietto retractor’s fenestrated blade is useful to fix the tip of the scapula through it (Figure 4). Adherences between chest wall and thoracic muscles must be released. Serratus muscle insertions are released anteriorly to facilitate exposure.
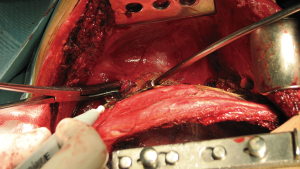
Once the chest wall is well exposed and all the adherences are completely free the 1st rib and the scalene muscles are visualized in upper thoracic outlet. It is important to be oriented at that point because of the lateral position of the patient. Posterior scalene muscle inserts to the posterior portion of the 2nd rib and is divided initially using a straight angle dissector (Figure 5). Secondly exposed muscle, inserted to the 1st rib, is the Middle Scalene and just beneath it runs the brachial plexus and subclavian artery. The middle scalene muscle needs to be carefully sectioned too.
Once the mid scalene muscle is identified the brachial plexus (posterior) and the subclavian artery (anterior) are exposed (Figure 6). Vascular affectation must be carefully checked although subclavian vessels are normally not involved in posterior Pancoast's tumors. Despite it is technically more demanding, resection and reconstruction of unexpected subclavian vessels invasion may be necessary. Anterior Pancoast’s tumors are further treated in a specific chapter.
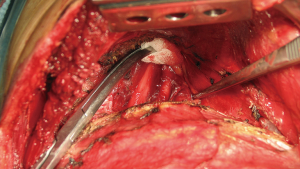
Chest wall resection is started anteriorly leaving a macroscopic wide margin—bigger than 1 cm (Figure 7). It is important to be sure about the margins because frozen section is not possible for bone invasion. Subcostal dissection is performed to surround and ligate the whole intercostal muscle. Distal ligature or clip is recommended to avoid infrequent but possible venous retrograde bleeding. After division of the intercostal muscle the superior border of the rib is exposed, then the rib is divided using a costotome. Resection of a 1 cm piece of rib is helpful to easily mobilize and resect the upper anterior borders of the chest wall.
1st rib is normally divided posterior to the subclavian artery and brachial plexus. In this step is when there is higher chance of unadvertised vascular lesion—we strongly recommend spending some time to dissect and secure the vessels for patient’s safety.
Using a dissector the first rib is completely surrounded ensuring sufficient oncologic margins. To divide the anterior border of the rib we prefer to use a Gigli saw instead of 1st rib costotome because it can be placed easily using the dissector and use it under direct vision assessment. The assistant has to protect vascular structures using a peanut while the surgeon saws the rib (Figure 8). After division the upper posterior border of the rib is cautiously divided while protecting brachial plexus from electrocoagulation.
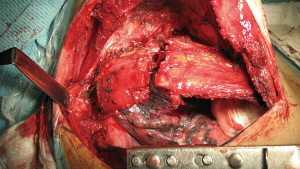
Paraspinous muscles are desinsherted and mobilized laterally to expose the costovertebral junction. Costotransverse ligaments are divided with electrocautery to expose vertebral transverse process and its junction with the rib (Figure 9). A straight periostotome is used to dislodge the ribs from inferior to superior. Pressure must be applied to anterior at the same time the assistant rotates the anterior rib border down to widen the space. It is important to avoid lever movement to posterior because it could damage the vertebra apophysis.
Once the vertebral facets are exposed the intercostal arteries and nerve roots are ligated or clipped to prevent bleeding or cerebrospinal fluid leakage. Transfixion non-absorbable stich may be necessary if there is no space for clips.
Disarticulation of the 1st rib is the most challenging one. Just beneath it arises the lower root of the brachial plexus-T1 and joins C8 just over the rib. Ligation and section of T1 could be done with no motor affection of the hand. However, all efforts must be focused to preserve C8 to avoid hand mobility impairment.
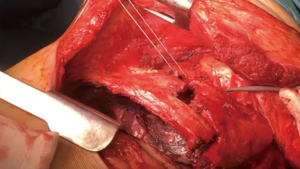
After tumor involved chest wall is completely released standard lobectomy is carried out through the wall defect. It is recommendable not to spread the remaining ribs to avoid added damage to the wall. Systematic mediastinal lymphadenectomy is done once the tumor is out the chest (Figure 10).
Meticulous hemostasis must be performed at the end of the surgery—specially revising arterial stumps and chest wall border—to prevent postoperative bleeding.
Direct closure of the chest wall is preferred in these surgeries because two main reasons. First, chest wall muscles and scapula disposition maintain the continuity in high-posterior chest wall defects. In consequence ventilation mechanics are not compromised. Second, synthetic exogenous materials are a risk factor for infection and colonization. In occasions, absorbable crossed stitches may be useful to maintain anterior rib borders in position.
Nevertheless, when the 5th rib has been resected chest wall continuity could be compromised. Scapula and posterior thoracic muscles give rigidity enough to allow normal respiratory movements and in consequence rigid reconstruction is not strictly necessary. In the other hand the discontinuity could affected scapular movement trapping it during abduction. Scapula compromise might lead to acute respiratory failure and contribute to chronic pain. In consequence we recommend chest wall reconstruction using a mesh fixed with interrupted stitches to the surrounding tissue when 5th rib is removed.
Tips and tricks
- Starting with the anterior border of the chest wall makes easier to perform the posterior disarticulation while applying pression/rotation.
- Dissection and control of the subclavian vessels is strongly recommended for patient’s safety in case of accident.
- It is easier and safer to cut the first rib using a Giggli saw than using the 1st rib costotome.
- Electrocoagulations should be avoided as much possible at the posterior and superior borders to prevent neuronal injury.
Complications
Trapped scapula
Chest wall reconstruction is recommended if costal resection under the 5th rib is necessary to avoid impaired scapula movement. Trapped scapula could compromise respiration and lead to severe respiratory failure.
Dehiscence of the thoracotomy
Individualized closure of all layers is mandatory to avoid chest wall dehiscence. Although this is essential after any surgery, this demanding and long procedure added to surgeon’s fatigue makes the closure even more important.
Cerebrospinal fluid leakage
It is important to identify and ligate the intercostal nerve during the disarticulation to avoid in adverted lesions. In case of CLF leakage reparation using materials that could migrate to the medullar canal must be avoided.
Vertebral artery injury
Preoperative assessment is important to know if Willis’ polygon is permeable in case the vertebral artery needs to be sacrificed for oncological or technical reasons.
Chilothorax
At the end of the surgery it is important to check for thoracic duct leakage. This is especially important in left sided resections because of anatomical disposition of the thoracic duct.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci, Alan D.L. Sihoe and Benedetta Bedetti) for the series “Open Thoracic Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.07.04). The series “Open Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rusch VW. Management of Pancoast tumours. Lancet Oncol 2006;7:997-1005. [Crossref] [PubMed]
- Panagopoulos N, Leivaditis V, Koletsis E, et al. Pancoast tumors: characteristics and preoperative assessment. J Thorac Dis 2014;6:S108-15. [PubMed]
- Rusch VW, Parekh KR, Leon L, et al. Factors determining outcome after surgical resection of T3 and T4 lung cancers of the superior sulcus. J Thorac Cardiovasc Surg 2000;119:1147-53. [Crossref] [PubMed]
- Detterbeck FC. Changes in the treatment of Pancoast tumors. Ann Thorac Surg 2003;75:1990-7. [Crossref] [PubMed]
Cite this article as: Boada M, Sánchez-Lorente D, Molins L. Posterior thoracic approach for Pancoast tumour resection. Shanghai Chest 2017;1:24.