Segmentectomies
Introduction
Definition
The word segment is derived from the Latin noun segmentum (“a piece cut off”) meaning part of larger structure. Bronchopulmonary segments are anatomical and functional units of lung parenchyma formed from subdivisions of each lobe. Each segment has a pyramidal structure with its apex at the hilum and its base on the surface of the lung supplied by (I) a segmental bronchus as tertiary branch of the bronchial tree; (II) a segmental branch of the pulmonary artery (as well as a bronchial artery); and (III) a segmental (± intersegmental) branch of the pulmonary vein together with lymphatics.
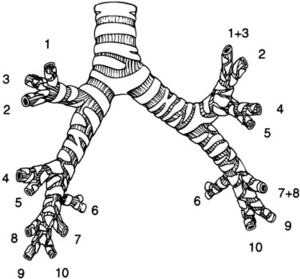
Segmental resection, or “segmentectomy”, describes a technique whereby part of the lung is excised along its anatomical landmarks based on bronchovascular anatomy. It involves division and closure of all individual bronchovascular segmental structures followed by dissection and removal of the lung parenchyma along the intersegmental planes. A thorough knowledge of the human lung anatomy is mandatory for any surgeon before embarking on resection of individual segments. There are 10 segments in the right lung (3 in upper lobe, 2 in middle lobe, and 5 in lower lobe) and 9 segments in the left lung (5 in upper lobe and 4 in lower lobe) (Figure 1).
Indications
Churchill and Belsey first described the technique of segmentectomy in 1939 in a patient with bronchiectasis (1). This type of resection was originally limited to patients with infectious diseases like bronchiectasis or tuberculosis as pneumonectomy was still the standard surgical resection for patients with lung cancer before 1950. This resection type should be preferred for benign conditions limited to a segment of the lung, but too centrally located for simple wedge excision; examples are bronchiectasis, pulmonary abscess, pulmonary sequestration, benign tumors (hamartoma), or centrally located metastatic lesions. In the modern era of lung surgery with widespread availability of antibiotics and antituberculous drugs, the number of segmentectomies performed for infectious lung processes has significantly decreased.
In 1973, Jensik and Faber published on a series of patients who underwent segmental resection for lung cancer questioning the standard of lobectomy already at that time (2). With the introduction of spiral and later on helical computed tomographic (CT) screening, more small-sized tumors and precancerous lesions are being found. This has led many surgeons to question the appropriateness of lobectomy for all cases of non-small cell lung cancer (NSCLC). In the last 2 decades, limited resection for early lung cancer, ranging from ground glass opacities (GGO), adenocarcinoma in situ (AIS), and minimally invasive adenocarcinoma (MIA), has continued to be an area of controversy with conflicting reports from non-randomized studies that compare survival after segmentectomy with those after standard lobectomy (3-5). In an effort to increase the level of evidence to support limited resection for small (≤2.0 cm) tumors, two prospective randomized studies were set up, one in the USA & Canada with an expected enrollment of 1,258 patients in 5 years (CALGB-140503) (6) and one in Japan with an accrual of 1,100 patients in 3 years (JCOG0802/WJOG4607L) (7).
There are two patient groups with early stage NSCLC that may benefit from segmentectomy as a surgical approach. The first group includes patients who can not tolerate a lobectomy because of poor pulmonary function test [forced expiratory volume in 1 second (FEV1) less than 50% of predicted] or have other lesions that will need to be resected. A lesser resection constitutes an alternative local cancer treatment now competing with other less invasive methods such as stereotactic body radiation therapy or radiofrequency ablation. The second group consists of patients who would tolerate a lobectomy, but in whom a curative resection is likely with a sublobar resection because of a small tumor size with negative lymph nodes. Until further evidence coming from the ongoing randomized trials, it is recommended that anatomical segmentectomy is currently reserved for fit patients with pure GGO lesions or partly (<25%) solid lesions (AIS, MIA) below 2 cm located in the peripheral third of the lung. These lesions are known to be non-invasive without malignant nodal spread (3-5). In case of segmentectomy, the tumor should be situated in the central aspect of a typical pulmonary segment and intraoperative frozen section of N1 and N2 nodes should have confirmed the T1aN0M0 status. In addition, the surgeon should always resect all intersegmental lymph nodes and check its tumor-free status. Otherwise the procedure should be converted into a lobectomy. Segmentectomy should not be performed when the tumor is close (<1.0 cm) to the intersegmental plane. Frozen section of the resection margin is therefore also recommended.
Approach
The original segmentectomy operation included an open approach as will be discussed further in this paper. As experience with video-assisted thoracic surgery (VATS) and robotic thoracic surgery (RATS) increases, more segmentectomies nowadays are being performed by these less invasive techniques, especially for easily resectable segments (S4–5 and S6) with reported reduced morbidity and 30-day mortality, and shorter hospital stay (8-11).
Terminology
It is important to understand the nomenclature that is being used in the literature to describe the number and the location of segments to be removed. The classical example and technically easiest surgical procedure is the removal of a single segment (monosegmentectomy) such as the apical segment of the lower lobe (S6), also called the Nelson or the Fowler segment. Another example is the removal of the two basal segments in the left upper lobe, equivalent to the right middle lobe. This procedure is not called lobectomy because of the absence (or incompleteness) of the horizontal fissure in the left lung, but rather bisegmentectomy (S4–S5), better known as lingulectomy. The above-described operations are more commonly performed, especially for T1aN0M0 NSCLC. Removal of the upper lobe, but sparing the lingula (the equivalent of the right upper lobe) is called trisegmentectomy (S1–S2–S3) or sometimes lingula-sparing upper lobectomy or culmenectomy. Finally, removal of 4 segments in the right lower lobe (S7–S8–S9–S10) or 3 segments in the left lower lobe (S7 & 8–S9–S10) sparing the apical segment (S6) is called basal segmentectomy.
Different terms are also being used in the literature to describe the extent of parenchymal resection, but they do not all match the same surgical procedure. A wedge resection sometimes referred to as an atypical segmentectomy because of its sublobar resection status, is not an anatomical resection and should not be confused with a typical, anatomical segmentectomy. This is an important distinction as a non-anatomical segmentectomy may have a negative impact on tumor-free resection margin, extent of lymph node sampling and excision, local recurrence, and overall survival in patients with early stage lung cancer. This mixture of atypical and typical segmentectomies has tainted the data in series used to recommend segmental resection as an adequate cancer operation. Extended segmentectomy describes a technique whereby the parenchyma is divided lateral to the intersegmental plane in order to have a wider resection margin in patients with a limited margin-to-tumor diameter ratio.
Preoperative assessment
The same principles of preoperative cardiopulmonary workup and evaluation to assess medical operability apply for segmentectomy as compared to more extended pulmonary resections like lobectomy or pneumonectomy. Cardiologic evaluation and lung function testing including carbon monoxide lung diffusion capacity (DLCO) are recommended in every patient undergoing pulmonary resection. Further exercise testing with oxygen consumption (VO2) should be performed in patients with FEV1 and/or DLCO <80% (12).
With regards to staging of suspicious or proven malignant lesions, combined positron emission tomography (PET)/CT scan nowadays has become the best radiographic and metabolic test to rule out extrapulmonary disease, to assess mediastinal and hilar lymph nodes, and to exclude other suspicious nodules in the lung. Endobronchial ultrasound (EBUS) and esophageal ultrasound (EUS) have become accurate and less-invasive tools for mediastinal staging that can be followed by video-mediastinoscopy to rule out false negative results in case of suspicious lymph nodes on imaging (13). CT or magnetic resonance imaging scan of the brain and CT or ultrasonography of the abdomen, especially in patients with proven adenocarcinoma are useful to complete a preoperative staging of NSCLC.
Operative techniques
Preparation
We favor an epidural catheter for postoperative pain control for all thoracotomy patients. An indwelling subpleural catheter for intercostal nerve block can also be recommended. This form of non-sedating analgesia is very important to decrease the risk of postoperative respiratory complications, especially in patients with poor pulmonary reserve scheduled for limited resection. A double lumen endotracheal tube or bronchial blocker is used for selective one-lung ventilation. The use of a pediatric bronchoscope introduced through the open lumen of the isolated lung may help to identify the correct bronchial anatomy by transilluminating the segmental airway to be divided whenever doubt.
Patient
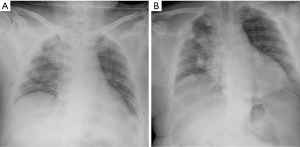
The patient described in this paper is a 66-year-old male with recent diagnosis of prostate cancer following rise in his prostate-specific antigen tumor marker. An incidental mass was discovered in the right kidney on abdominal CT scan during further oncologic screening of his prostate cancer. Simultaneously, chest CT scan revealed a nodular mass in the posterior segment (S3) of the right upper lobe (Figure 2), proven to be a synchronous metastasis from a clear cell carcinoma on EBUS-guided needle biopsy. Six weeks following a laparoscopic right nephrectomy for a clear cell carcinoma (pT3N1M1), an anatomical posterior segmentectomy (S3) in the right upper lobe was performed via open thoracotomy. Pathologic examination revealed a metastasis (2.2×2×2 cm) from a clear cell carcinoma with negative margins and negative hilar and mediastinal lymph nodes. Patient had an uneventful recovery and was discharged home on the 6th postoperative day with a clear chest-X ray (Figure 3).

Exposition
The authors favor an anterior approach as is currently the standard access for all our open and VATS pulmonary resections and for double-lung transplantations. Pulmonary structures will need to be divided working from anteriorly towards the posterior aspect of the lung. Once the targeted lung part is being removed, the larger airways and the posterior mediastinum become exposed allowing additional airway or arterial sleeve resection as well as mediastinal lymph node dissection whenever indicated.
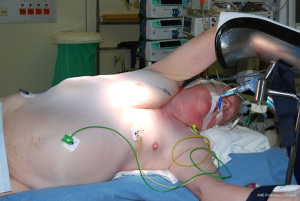
The patient is placed in semilateral (45°) decubitus position. The body is fixed on the table using a vacuum matrass with the ipsilateral arm supported and protected on an arm rest and the contralateral arm positioned on an arm rest 90° to the body (Figure 4). The table is tilted slightly in an anti-Trendelenburg position with both legs lifted up for better venous return. During the operation, the table can always be turned towards the surgeon or the first assistant as needed. Medical images of the lesion(s) to be resected are projected on a large computer screen for intraoperative consultation. Patient’s intraoperative parameters are projected on the video screens for cardiopulmonary monitoring by the surgeon whenever needed during one-lung ventilation or clamping of the ipsilateral main pulmonary artery.
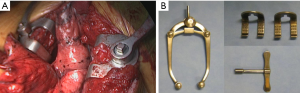
The chest is entered via a submammary incision through the fourth intercostal space. This incision results in a muscle-sparing thoracotomy as the pectoralis major and the anterior portion of the serratus anterior are the only muscles that need to be divided. The remaining extrathoracic muscles can be lifted with a Richardson retractor while the intercostal muscles are incised from anteriorly (sparing the mammary vessels whenever possible) all the way down towards the level of the sympathetic chain posteriorly. By doing so, the ribs can be sufficiently spread for intercostal access as needed. We prefer to use the Price-Thomas rib spreader rather than the classical Finochietto retractor as this instrument stabilizes the ribs much better while opening the chest in a V-shape. The valves of the spreader can be adjusted according the thickness of the subcutaneous fatty and muscular layers avoiding the use of other soft tissue spreaders (Figure 5). Occasionally, a second Price-Thomas spreader is needed to better open the incision anteriorly by luxating the cartilaginous bone at its junction with the sternum.
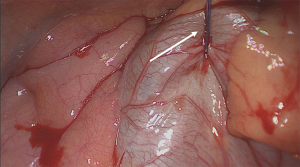
The next and most important step is to place a heavy pericardial suture anteriorly to the phrenic nerve and through the subcuticular skin layer. By tying this suture, the heart protected by the pericardium will be lifted up substantially giving a superb exposure to the hilar vessels while facilitating access to the posterior mediastinum. Meanwhile the heart remains in a stable position avoiding need for further traction and compression intraoperatively (Figure 6).
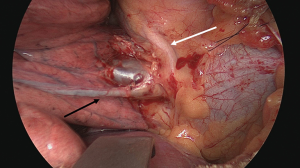
On entrance of the chest, careful inspection of the pleural cavity and palpation of the lung is conducted to rule out other, unexpected pathology. If no tissue diagnosis is known preoperatively and if the lesion is located peripherally and clearly within a segment, a wedge excision can be performed first for intraoperative frozen section. In case of a more central lesion within the segment, a tru-cut needle biopsy can be done first or directly a segmentectomy in a fit patient in case of high tumor suspicion. As previously stated, adequate hilar and intersegmental lymph node sampling and evaluation of the resection margin are needed to ensure the procedure is correctly executed from an oncologic standpoint. Mediastinal and hilar nodal dissection can be done as needed after the segment has been removed.
Operation
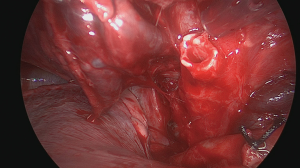
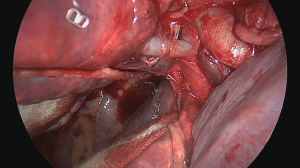
The initial step is to identify the intrathoracic anatomy and the hilar structures (Figure 7) (14). In our patient, the upper lobe vein was identified first. After cleaning the hilar fad, three major venous branches showed up. One small branch drained venous blood coming from the middle lobe; the two other branches came from the upper lobe (Figure 8). To identify the individual segmental anatomy, opening of the fissure is needed prior to transection of any vessels. First, the interlobar pulmonary artery is identified in the fissure and a tunnel is created connecting with the hilar area anteriorly.

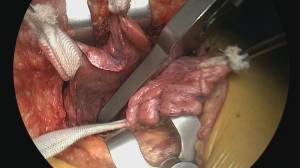
The horizontal fissure is then divided using a linear cutter (Figure 9) (15). We prefer to use the ILATM 100 re-usable stapler (Medtronic, Jette, Belgium) as this device is the cheapest stapler available with usually only one cartridge needed to transect the parenchymal bridge (Figure 10). Occasionally, a second cartridge on the ILATM 52 is used to complete parenchymal transection. In theory, for posterior segmentectomy in the upper lobe, the horizontal fissure can remain intact. However, interlobar anatomy with the individual venous and arterial branches to the three lobes becomes clearer after parenchymal division avoiding erroneous transection. Attention is needed not to damage the middle lobe artery when dividing the horizontal fissure. The next step is to divide the oblique fissure between the apical segment of the lower lobe and the basis of the upper lobe posteriorly. A second tunnel is therefore created from the interlobar space between the upper lobe bronchus and the intermediate bronchus posteriorly towards the pulmonary artery in the fissure. Sharp dissection with a scissor immediately beyond the interlobar artery guided by the finger coming from posteriorly is helpful to safely create this tunnel. The oblique fissure is then divided with the same stapler (one cartridge on the ILATM 100 usually suffices). Careful attention should be given not to damage the vascular supply going to the apical segment of the lower lobe (S6) as this arterial branch may come off from the interlobar pulmonary artery rather high!

With both fissures now completely divided, identification of the segmental vasculature becomes clearer (Figure 11). The vein draining blood from the posterior segment crosses the artery in the fissure. It should be divided with suture or ligature distal to any other side veins draining the middle lobe or other segments of the upper lobe. Usually, the segmental artery to the posterior segment (S3) takes off from the interlobar artery in the fissure, also known as the posterior ascending artery (Figure 12). Depending on its size, this artery can be ligated and clipped or sutured (Figure 13) (16). Proximal control by encircling the pulmonary artery with a tape can be useful in case vascular anatomy is unclear or when fissure planes are inflamed or scared, but this is not our routine practice. After division of the vasculature, the take off of the upper lobe bronchus becomes clear (Figure 14). The three segmental bronchi going to the individual upper lobe segments can then be identified by dissecting the upper lobe bronchus more distally (Figure 15) (17). Gentle traction on the segment to be resected with an atraumatic clamp may help to expose the bifurcation of segmental bronchi as these may be hidden more deeply into the lung parenchyma. The segmental bronchus (S3) to the posterior segment usually runs behind and parallel to its segmental artery.
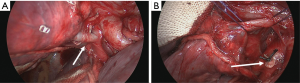

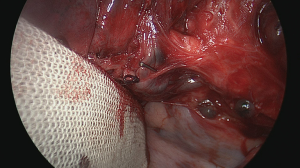

Prior to transecting the airway, the lung should be reventilated with the segmental bronchus clamped in order to verify the correct anatomy (Figure 16) (18). The virtual fissure running between the posterior segment and the two remaining (anterior and apical) segments will then become more clear as the atelectatic parenchyma will no longer ventilate or will be aerated more slowly through collateral ventilation across the intersegmental plane (Figure 17) (19). The segmental bronchus is then divided with a stapler or a bistouri (Figure 18). Gentle traction on the distal bronchial stump will open up the hilum of the segment giving better exposure to the excluded parenchyma.


The final step is transection of the intersegmental plane to exclude the involved segment (S3) from the remaining segments (S1 and S2) (Figure 19) (20). This can be done with the same linear stapler or with electrocautery while the remaining part of the lung is being ventilated with the bronchial stump clamped. Finally, in case no stapler was used for airway closure, the bronchus has to be sutured (Figure 20) (21). We prefer a manual closure using two over-and-over running polydioxanone 4/0 absorbable monofilament sutures after folding the membranous part inside the lumen of the bronchus and bringing the cartilaginous rings together according to the technique previously described by the Dutch surgeon Klinkenberg (Figure 21).


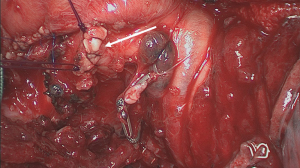
Completion
The remaining lobe is then tested under water while ventilated to identify any remaining air leak from the bronchus or the parenchyma. The small bronchial stump can be covered with any fatty or pleural tissue flap surrounding the bronchus or even with the remaining stapler line after parenchymal division. In order to avoid hilar torsion, the remaining segment(s) in the upper lobe can be approximated to the middle and/or lower lobes using a parenchymal suture on a vascular clamp or by approximating and fixing both staple lines left over after transection of the horizontal or oblique fissures.
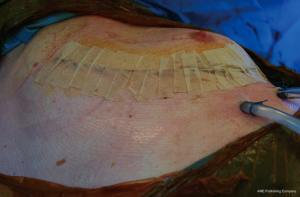
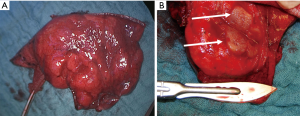
After careful check for any bleeding, the chest is being closed. We prefer to insert two large drains size CH 28; one curved basal on the diaphragm and one straight apical with the tip in the remaining cavity. The ribs can be approximated with one or two heavy pericostal sutures of absorbable material (polyglyconate 2 or polydioxanone 2). The remaining wound is closed in layers in the usual manner (Figure 22). The resected specimen is then sent to the pathology department for further histological and immunological examination (Figure 23).
Comments
Postoperative care
Postoperative care after segmentectomy is comparable to other patients undergoing pulmonary resection. We do not put the drains on suction routinely unless there is an increasing pneumothorax or subcutaneous emphysema as a result of a large airleak. The focus of postoperative care is directed towards adequate analgesia and pulmonary toilet. A dedicated pain team is visiting our patients on the ward daily to check the patient-controlled analgesia pumps. Dedicated physiotherapy, early ambulation, regular portable chest X-rays, and a low threshold for bronchoscopy and antibiotics are all helpful to minimize the risk of pulmonary complications.
Specific complications
The morbidity and mortality after segmentectomy is comparable with patients after lobectomy in most reported series. After segmentectomy, residual space problem is less frequently seen compared to lobectomy. However, the postoperative course can be complicated by atelectasis of remaining segments as a result of sputum impaction or disturbed bronchial anatomy or by persistent air leaks, especially if no stapling devices were used to divide the intersegmental plane in an attempt to preserve maximum volume of the remaining lung. A residual pseudotumor can sometimes be seen on chest X-ray as a result of a local hematoma filling the remaining cavity of the removed segment or developing in adjacent lung parenchyma. A progressive lobar infiltrate unique to the remaining lobe in a septic patient may be the first sign of a lobar infarct. Re-exploring the patient may be indicated after computer tomographic and bronchoscopic work-up.
Caveats
The spatial approach and the order of dividing the individual bronchovascular structures may well vary according to the individual segment(s) to be removed, but the overall principles remain the same. The easiest procedures are the removal of the superior segment of the lower lobe (S6), the lingular segments (S4 + S5), and the basilar segments of the lower lobe (S7–S10), while the individual segments in the upper lobe (S1, S2, S3) and lower lobe (S7, S8, S9, S10) are more challenging (Figure 1).
Specific segments (S4–S5–S6) have an individual central vein that can be ligated or clipped while the other segments have veins that run closer to the periphery of the segment and can not be identified until after dissection of the intersegmental plane has started and drainage into the superior or inferior venous trunks become visible. Sometimes, these veins will not become identifiable until after stapling of the parenchyma. It is however important to identify the anatomy of the venous trunks carefully before dividing segmental vessels to avoid inadvertent ligation of veins draining blood from adjacent segments. This may result in venous thrombosis, lobar infarct, and potentially disastrous complications postoperatively.
Identifying the appropriate plane for parenchymal division can be challenging, especially when doing VATS segmentectomy. Differential ventilation of the individual segments by clamping the segmental bronchus before (or after) full inflation can help to better delineate the intersegmental plane. In patients with severe COPD, collateral ventilation between adjacent segments through the pores of Kohn may trouble the identification of the intersegmental plane.
Two techniques have been described for parenchymal division: the open and stapled division.
In the open technique, the intersegmental plane is teared by traction on the stump of the transected segmental bronchus with a clamp while the rest of the lung is well ventilated. Sharp and blunt finger dissection may help to open the intersegmental plane. Diathermy, harmonic scalpel, or small vascular clips are used to ensure hemostasis as more blood loss may be expected. Small air leaks can be over sewn with fine sutures. In addition, the raw surface of the adjacent segment denuded from its visceral pleura can be covered with pleural or pericardial fat flaps for additional hemostasis and aerostasis. Care has to be taken to avoid compression or kinking of remaining bronchovascular structures caused by the flap. This might end up in a non-functional lobe eliminating the benefit of segmentectomy versus lobectomy. The advantage of the non-stapled technique is that re-expansion of the adjacent parenchyma is maximally filling the empty segmental space, but it carries a higher risk of prolonged air leaks from unidentified bronchioli not sutured or clipped resulting in a small broncho-pleural fistula.
In the closed technique the virtual fissure is compressed and cut with the aid of a linear stapler. We prefer a re-usable linear cutting device with a stapling length of 10.0 cm. Usually more than 1 cartridge is needed. This technique results in a better pneumostatic control in the remaining lung, but it comes at the expense of volume loss as the visceral pleural layers are stapled together when closing the device. The remaining parenchyma is then somewhat trapped by the individual staplers blocking re-expansion of the lobe until its maximum volume.
Summary
Segmentectomy has been part of the thoracic surgeon’s armamentarium by nearly 80 years. In the last 4 decades, the indication for segmentectomy has shifted from a procedure to remove a destroyed segment after infection towards a surgical option for patients with very early, peripheral (Stage IA ≤2.0 cm) NSCLC or for patients with limited cardiopulmonary reserve with a favorable sized and located NSCLC without lymph node involvement. Retrospective and single-institutional reports suggest that the outcome in carefully selected patients with small (≤2.0 cm) peripheral tumors (mainly adenocarcinoma) is excellent after anatomic segmentectomy and comparable to lobectomy. Nevertheless, before segmentectomy can become the standard resectional procedure for early NSCLC, we need to await the results of two ongoing randomized controlled trials. VATS or RATS approach for sublobar resection has proven to be feasible achieving excellent survival results comparable to open segmentectomy, but with shorter hospital stay and lower pulmonary morbidity. Proponents of this approach believe it may well become the future of surgery for lung cancer (22).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Churchill ED, Belsey R. Segmental pneumonectomy in bronchiectasis: the lingula segment of the left upper lobe. Ann Surg 1939;109:481-99. [Crossref] [PubMed]
- Jensik RJ, Faber LP, Milloy FJ, et al. Segmental resection for lung cancer. A fifteen-year experience. J Thorac Cardiovasc Surg 1973;66:563-72. [PubMed]
- Hennon M, Landreneau RJ. Role of Segmentectomy in Treatment of Early-Stage Non-Small Cell Lung Cancer. Ann Surg Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Sesti J, Donington JS. Sublobar Resection: Ongoing Controversy for Treatment for Stage I Non-Small Cell Lung Cancer. Thorac Surg Clin 2016;26:251-9. [Crossref] [PubMed]
- Landreneau RJ, D’Amico TA, Schuchert MJ, et al. Segmentectomy and Lung Cancer: Why, When, How, and How Good? Semin Thorac Cardiovasc Surg 2017;29:119-28. [Crossref] [PubMed]
- Comparison of Different Types of Surgery in Treating Patients With Stage IA Non-Small Cell Lung Cancer. Available online: https://clinicaltrials.gov/ct2/show/record/NCT00499330
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Yang CF, D’Amico TA. Open, thoracoscopic and robotic segmentectomy for lung cancer. Ann Cardiothorac Surg 2014;3:142-52. [PubMed]
- Atkins BZ, Harpole DH Jr, Mangum JH, et al. Pulmonary segmentectomy by thoracotomy or thoracoscopy: reduced hospital length of stay with a minimally-invasive approach. Ann Thorac Surg 2007;84:1107-12; discussion 1112-3. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Pennathur A, et al. Anatomic segmentectomy for stage I non-small-cell lung cancer: comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg 2009;138:1318-25.e1. [Crossref] [PubMed]
- Ghaly G, Kamel M, Nasar A, et al. Video-Assisted Thoracoscopic Surgery Is a Safe and Effective Alternative to Thoracotomy for Anatomical Segmentectomy in Patients With Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:465-72; discussion 472. [Crossref] [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemoradiotherapy). Eur Respir J 2009;34:17-41. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Frick AE, Van Raemdonck D. The hilar anatomy after opening the chest. Asvide 2017;4:355. Available online: http://www.asvide.com/articles/1669
- Frick AE, Van Raemdonck D. The division of the horizontal fissure between the upper lobe and the middle lobe anteriorly. Asvide 2017;4:356. Available online: http://www.asvide.com/articles/1670
- Frick AE, Van Raemdonck D. The posterior ascending artery and vein (S3) ligated and divided. Asvide 2017;4:357. Available online: http://www.asvide.com/articles/1671
- Frick AE, Van Raemdonck D. The bronchial anatomy after complete division of the oblique fissure between upper and lower lobe. Asvide 2017;4:358. Available online: http://www.asvide.com/articles/1672
- Frick AE, Van Raemdonck D. Clamping of the posterior segmental bronchus (S3) prior to reventilation. Asvide 2017;4:359. Available online: http://www.asvide.com/articles/1673
- Frick AE, Van Raemdonck D. Reventilation of the remaining upper lobe after clamping the posterior segmental bronchus. Asvide 2017;4:360. Available online: http://www.asvide.com/articles/1674
- Frick AE, Van Raemdonck D. The virtual fissure between the anterior and posterior segment is transected using a re-usable linear stapler. Asvide 2017;4:361. Available online: http://www.asvide.com/articles/1675
- Frick AE, Van Raemdonck D. This posterior segmental bronchus prior to suturing. Asvide 2017;4:362. Available online: http://www.asvide.com/articles/1676
- Swanson SJ. Video-assisted thoracic surgery segmentectomy: the future of surgery for lung cancer? Ann Thorac Surg 2010;89:S2096-7. [Crossref] [PubMed]
Cite this article as: Frick AE, Van Raemdonck D. Segmentectomies. Shanghai Chest 2017;1:28.