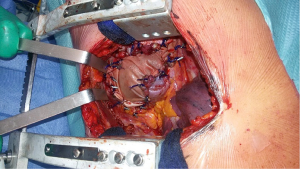
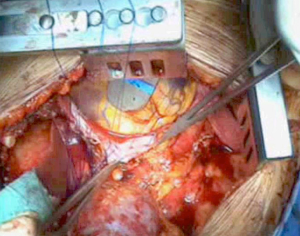
Extrapleural pneumonectomy
Introduction
Malignant pleural mesothelioma (MPM) is the major cause of death in patients exposed to asbestos. If not treated, survival of patients with MPM is dismal (1). Surgery or chemotherapy or radiotherapy alone are not effective. Although there is no defined standard treatment for MPM patients, most studies in the literature support the use of curative intent surgery in the setting of combined therapy (1-6).
Prognostic factors associated with longer survival are epithelial histology, female gender, and earlier stage. Women experienced longer survival compared with man, but this finding has been more consistent for younger women and those with epithelial tumors.
Surgery represents the main modality in treating MPM. Two operations have been developed in this context: extrapleural pneumonectomy (EPP), and radical or extended pleurectomy/decortication (P/D). The former, consists in the en bloc resection of the lung, parietal and visceral pleurae, diaphragm, and pericardium. P/D include the resection of both the parietal and visceral pleurae, with diaphragmatic and/or pericardial removal in case of their direct neoplastic invasion; in this case, the lung is preserved.
Since Butchart (7) reported the first report of EPP for MPM in seventies, the rate of perioperative mortality due to this operation dropped from 31% to 3.4%. Butchart emphasized that this technique may be indicated for certain types of tumors and, thus, adequate preoperative cardiopulmonary evaluation and careful preoperative management of patients were mandate.
A detailed preoperative evaluation is necessary in order to determine whether complete resection is feasible and whether the patients has sufficient physiological reserve to tolerate this very invasive procedure.
The extend of the spread of MPM is assessed initially by imaging studies. Computed tomography (CT) of the chest and upper abdomen is the primary investigation in assessing the extent of the tumor. It can identify signs of MPM spread, which are indicative of unresectable disease as chest wall invasion, the involvement of the full thickness of the diaphragm, the presence of metastatic spread in the peritoneum o in the contralateral lung or pleura.
PET CT (Figure 1) is also routinely used for evaluating nodal and distant metastases. The level of PET avidity of the mesothelioma has been shown to correlate with survival, with great avidity associated with lesser survival. Enlarged and/or PET-positive mediastinal nodes are evaluated with endobronchial ultrasonography (EBUS) and/or, in some centers, by cervical mediastinoscopy.
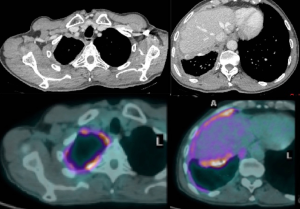
Chest MRI is sometimes performed to study more precisely invasion of the tumor o the chest wall or diaphragm. In case of transdiaphragmatic extension of tumor and/or ascites should be evaluated with staging laparoscopy because intrabdominal invasion would preclude surgical resection.
Preoperative evaluation of the cardiopulmonary reserve is of the utmost importance in patients for whom EPP is being considered. Respiratory evaluation includes spirometry, diffusion capacity, and quantitative ventilation/perfusion scan. Cardiac tests (stress test and an echocardiogram with Doppler estimation of pulmonary artery pressure) should also be performed to evaluate cardiac function. The presence of pulmonary hypertension is a contraindication to perform EPP.
Operative technique
Preparation and perioperative management
Preparation includes placement of (I) arterial line for monitoring arterial pressure which may rapidly change during the procedure; (II) venous line (central one) for rapid infusion of fluids; (III) epidural catheter for postoperative pain control.
Intubated is performed with a double-lumen to assure one-lung ventilation. A naso-gastric tube is inserted to allow the intraoperatively identification of the esophagus and to decompress the stomach perioperatively avoiding life-treating aspiration.
Exposition
The patient is placed in the lateral position and the operative sterile field should include the hemithorax having as limits the axilla, the neck, the sternum and posteriorly the spine. During operation, the table will change its position according to the different phases of the operation.
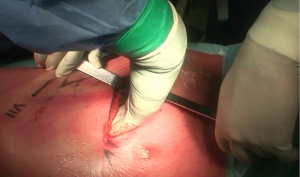
During operation, the surgeon is placed to the patient’s back and the first assistant on the other side of table. The surgical procedures are performed via a double muscle sparing lateral thoracotomy. Skin incisions are made in the 4th and in the 7th intercostal space, respectively (Figure 2). Prior incisions are removed.
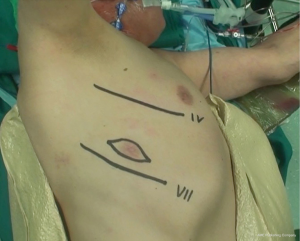
Operation
The first step of the intervention after skin incision in the 4th intercostal space is the incision of intercostal muscles exposing the parietal pleura. Identification of the parietal pleural is crucial at this stage. Whilst elevating intercostal muscle with forceps, the extrapleural plane is developed, initially with scissors or a dissector, and next with blunt finger dissection (Figure 3).
A big and a smaller finocchietto retractors are placed to effectively keep the wound open, tether the scapula, and to ensure a wide exposure of the pleural cavity. Rib spreading with retractors must be extended gradually as the dissection of the parietal pleura proceeds to avoid excessive strain that could cause a tear in the pleura and the lung. Dissection of the parietal pleura from the endothoracic fascia is conducted anteriorly, posteriorly, superiorly, and inferiorly. The limits of dissection are, posteriorly, the azygous vein on the right and the aorta on the left in order to prevent dramatic venous and/or arterial lesions.
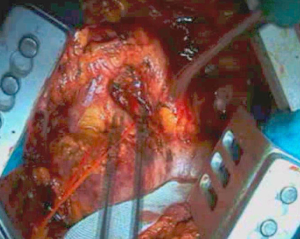
Dissection proceeds over the cupola of the pleura (Figure 4) near subclavian vessels. In this area, it is important to try to identify the phrenic nerve and the internal mammary vessels which should be clipped or ligated. Once apical dissection is completed, sponge gauzes are used to pack this space. Pleural dissection proceeds in the posterior mediastinum avoiding esophageal damage. At this step, the esophagus is easily identified by palpating the nasogastric tube previously positioned. Moreover, during this step, the assistant retracts anteriorly the pleural sac allowing the surgeon to dissect the parietal pleura from the esophagus.

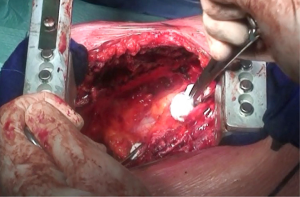
As the dissection is continued down the superior mediastinum, the superior vena cava on the right (Figure 5) and the aortic arch on the left are at risk (Figure 6). During this step, it is important to pay attention to the anterior mediastinum and pericardium (Figure 7). The parietal pleura and lung are moved posteriorly and the pericardium is opened in the anterior portion to check the presence of intrapericardial infiltration. The absence of involvement of mediastinal structures, allows to continue with the pericardial incision towards the inferior portion of the diaphragm.
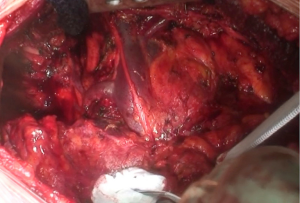
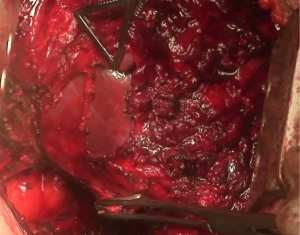
At this point, a second thoracotomy is performed at the seventh intercostal space. Once the intercostal muscles are incised and the inferior parietal pleural is identified, the dissection of the parietal pleural proceeds superiorly to encounter the previous dissected pleura. Then the dissection continues posteriorly, anteriorly and inferiorly toward the diaphragm. Here, the diaphragm is bluntly dissected from the chest wall with the fingers. During these maneuvers, caution should be used on both sides: on the right, for the inferior vena cava; on the left, a small portion of the diaphragm is retained to reduce the risk of abdominal organs herniation. Dissection is usually easier at the muscular part of the diaphragm and more difficult in the central tendon area (Figure 8). Generally, this stage of the operation is tedious and time-consuming, and needs a lot of patience. During the dissection, the peritoneum is entered identification the main veins originating from the lever. It is not necessary to close the peritoneum. The pericardial incision is continued to complete the pericardial resection.
Hilar structures are then approached through incision in the 4th intercostal space. Superior and inferior pulmonary veins are isolated and resected intrapericardially by endostaplers (30 mm, white reload). Right pulmonary artery is isolated and resected intrapericardially, while, on the left, pulmonary artery is generally divided extrapericardially due to the short intrapericardial arterial segment by endostaplers (45 mm, white reloads).
The mainstem bronchus is dissected and resected near the carina with a bronchial stapler. The specimen, consisting of the lung within the parietal pleural envelope, hemidiaphragm, and part of the pericardium is removed en bloc from the chest (Figure 9).
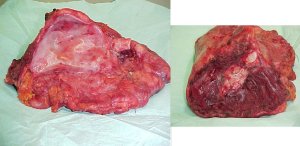
Lymphadenectomy of the mediastinal stations is routinely performed.
The bronchial stump can be buttressed with a vital pedunculated flap (thymic tissue or omentum). Accurate haemostasis of the entire chest wall is then performed by means of argon beam coagulation. On the left side, due to the high risk of chylothorax, a massive ligation of the tissue near the phreno-pericardial angle, next to the esophagus should be performed.
Diaphragm and pericardium are reconstructed with different types of heterologous patches [Gore-tex, polytetrafluoroethylene (PTFE), Marlex, Vicryl, and bovine pericardium]. After the initial use of a Marlex/Gore-tex mash for diaphragmatic reconstruction, and Gore-tex for pericardial reconstruction, the material actually used by the authors for reconstruction of both diaphragm and pericardium is bovine pericardium patches.
The diaphragm is reconstructed with 1 or 2 20×10 cm patches sutured in the inner portion of the chest wall with separated non-absorbable stitches (1 Polypropylene) (Figure 10) which fixe the patch to the chest wall (to the tenth rib posteriorly and at the eighth or ninth rib anteriorly and laterally).
Next, the pericardium is reconstructed using a single bovine pericardium patch (Figure 11) which is fenestrated to avoid cardiac impairment due to effusion and tamponade. The patch is sutured to cut edge of the pericardium circumferentially using separated non-absorbable stitches (3-0 Polypropylene).
Haemostasis should be checked carefully and all sites of oozing controlled with cautery and argon beam coagulation.
After extrapleural dissection a greater fluid production together with an increased risk of bleeding or chylothorax may occur. Usually the endothoracic cavity may fill rapidly and may produce a more mobile mediastinum. Thus, a chest tube without suction with balanced drainage is necessary. We usually place one 32Ch single tube into the chest connected to a balanced reservoir.
The two thoracotomies are closed n the standard manner. Particular attention is paid to obtaining a watertight intercostal closure to avoid leakage of fluid through the chest wall and pleural cavity infection.
Postoperative management
EPP should be performed in high specialized and high volume centers in which all the surgical team including anaesthetists, nurses, and critical care team are experienced in managing of these surgical patients.
The patient should be rapidly extubated to reduce the risk of damage on the bronchial stump with positive pressure and strictly monitored postoperatively in the ICU.
Postoperative management includes management of fluid status, physiotherapy, and early mobilization. Antithrombotic prophylaxis with low-molecular weight heparin is routinely administered.
Some complications may occur after EPP and these are usually related to injuries to anatomic structures during intervention: lesion of the recurrent laryngeal nerve may cause vocal cord paralysis and poor cough and/or aspiration. Damages of the sympathetic nerves may occur after extrapleural dissection; this may cause postoperatively mild but refractory vasoplegia. This effect usually responds to oral vasoconstrictors that can be reduced in the postoperative period. In this case, administration of excessive fluids to regulate this benign source of hypotension should be limited to avoid other consequent pulmonary and cardiac complications (atrial fibrillation, pulmonary edema) in these patients with only one lung remaining.
Rare complications after EPP include cardiac or gastric herniation, myocardial infarction, chylothorax, and pulmonary embolus. The most feared complication of this surgery is the bronchopleural fistula that may occurs weeks to months after initial intervention. It should be rapidly evaluated and treated to avoid fatal consequences.
Comments
Cytoreductive surgery is the cornerstone of the multimodality approach to treating patients with MPM. Maximal cytoreduction is critical to extend long-term survival. EPP can achieve this result in most cases of tumor confined to the ipsilateral chest.
In the Italian Multicentric retrospective study (5) on 518 EPPs for MPM, major morbidity was 26% and 90-days mortality rate was 6.9%. The median overall survival was 18 months with a 1-, 2- and 3-year overall survival of 65%, 41%, and 27%, respectively. This study confirmed that female gender with epithelial tumor who received induction therapy had the best prognosis after EPP.
The physiological stresses following EPP are numerous and appropriate patient selection facilitated by through preoperative testing is mandatory. Likewise, the diagnosis of postoperative complications must be pursued vigilantly to facilitate rapid treatment. Many of these complications can be prevented with careful attention to detail in managing the physiologic consequences of EPP.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sugarbaker DJ, Wolf AS. Surgery for malignant pleural mesothelioma. Expert Rev Respir Med 2010;4:363-72. [Crossref] [PubMed]
- Wolf AS, Richards WG, Tilleman TR, et al. Characteristics of malignant pleural mesothelioma in women. Ann Thorac Surg 2010;90:949-56. [Crossref] [PubMed]
- Weder W, Kestenholz P, Taverna C, et al. Neoadjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. J Clin Oncol 2004;22:3451-7. [Crossref] [PubMed]
- Flores RM, Krug LM, Rosenzweig KE, et al. Induction chemotherapy, extrapleural pneumonectomy, and postoperative high-dose radiotherapy for locally advanced malignant pleural mesothelioma: a phase II trial. J Thorac Oncol 2006;1:289-95. [Crossref] [PubMed]
- Spaggiari L, Marulli G, Bovolato P, et al. Extrapleural pneumonectomy for malignant mesothelioma: an Italian multicentric retrospective study. Ann Thorac Surg 2014;97:1859-65. [Crossref] [PubMed]
- Casiraghi M, Maisonneuve P, Brambilla D, et al. Induction chemotherapy, extrapleural pneumonectomy, and adjuvant radiotherapy for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Butchart EG, Ashcroft T, Barnsley WC, et al. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976;31:15-24. [Crossref] [PubMed]
Cite this article as: Galetta D, Spaggiari L. Extrapleural pneumonectomy. Shanghai Chest 2017;1:32.