
Randomized controlled trial on minimally invasive versus open esophagectomy for esophageal cancer: short and long-term outcomes
The incidence of esophageal cancer has been increasing over the past two decades (1). Despite improvement in treatment options, such as chemotherapy and radiotherapy, esophagectomy with regional lymph node dissection remains the mainstay of curative modality for patients with localized thoracic esophageal cancer. Morbidity is a major concern during the follow-up period because of the invasive nature of esophagectomy and the complex operative procedures involved. Long thoracic and abdominal incisions and one-lung ventilation during esophagectomy are thought to be partly responsible for the high surgical invasiveness and subsequent respiratory complications of this procedure. On the other hand, a thoracoscopic approach, which could reduce the length of skin incision, has been attracting attention as a minimally invasive esophagectomy (MIE). By the late 1990s, several surgeons had performed and demonstrated the safety and feasibility of the technique (2,3). After these exploratory investigations, reports from large single-center studies began to reveal improvements in the surgical outcomes of MIE (4). Meta-analyses using individual institutional reports showed that compared with open esophagectomy (OE), MIE was associated with less operative blood loss, shorter length of intensive care unit and hospital stays, and reduced incidence of postoperative respiratory complications (5,6). On the other hand, results from several nationwide database analyses have been disappointing and demonstrated that MIE did not reduce postoperative respiratory complications and had higher reoperation or reintervention rates (7,8). However, these unexpected results of the nationwide database analyses may be attributable to the inclusion of a wide range of patients, surgeons, and hospitals. Therefore, we have recognized the necessity of a prospective study to determine the lower invasiveness and improved quality of life (QOL) associated with MIE, compared with OE.
Straatman and colleagues conducted a multi-center, open-labeled, randomized controlled trial to compare the long-term outcomes between MIE and OE (9). Consistent with the above descriptions, the aim of this study was to determine the advantages of MIE in the management of patients who underwent esophagectomy for esophageal cancer. This study included 115 patients from five European hospitals and who were randomly assigned to the OE (n=56) or MIE (n=59) group. In the TIME trial, Biere et al. previously described the short-term outcomes and clear benefits of MIE, such as less postoperative pulmonary complications, shorter hospital stays, and a better short-term QOL (10). In this follow-up study of the TIME trial, the OE and MIE groups did not differ in terms of three-year overall survival rates [40.4%±7.7% vs. 50.5%±8%, respectively; P=0.207; hazard ratio (HR) 0.883 (0.540 to 1.441)], and disease-free survival rates [35.9%±6.8% vs. 40.2%±6.9%, respectively; P=0.602; HR 0.691 (0.389 to 1.239)]. Multivariate analyses for both overall and disease-free survival rates, with correction applied for stage of disease; gender; and age, revealed no differences between the two esophageal procedures.
As Straatman et al. investigated, MIE has the potential to allow faster recovery to normal function and to decrease morbidity of patients after esophagectomy (11). Luketich et al. conducted a prospective, phase II, multicenter trial and demonstrated the short-term feasibility and safety of MIE (12). However, multicenter randomized trials have not been reported until quite recently because of the surgeons’ level of experience and the diversity of operative techniques used for MIE. Standardization of surgical techniques and perioperative management had been considered as the key in conducting a multicenter MIE trial (13). Recent studies have demonstrated a volume outcome relationship for esophageal surgery; in particular, morbidity and mortality significantly decreased in high-volume hospitals (14). These improved outcomes in high-volume hospitals partly depend on thorough perioperative management by a multidisciplinary team using agreed written protocols throughout the patient’s hospital stay. To prevent such bias, only surgeons with experience on at least 10 MIE procedures and sufficient skills were allowed to participate in the TIME trial (10). One surgeon who was experienced on MIE was asked to proctor the skill of another surgeon on surgical video before starting the trial. Furthermore, to prevent institutional bias, only the hospitals with high volume (i.e., more than 20 esophagectomy procedures per year) were allowed to participate in the trial. Consequently, each participating center included an average of 23 patients. These restrictions on participating surgeons and hospitals are considered to be critical when conducting this kind of radical trials on surgical outcomes.
In the TIME trial, the MIE group achieved better short-term outcomes, such as less postoperative pulmonary complications, shorter hospital stays, and a higher QOL, compared with the OE group (10). Another supplementary study of the TIME trial showed that the higher QOL of the patients in the MIE group persisted until one-year follow-up (15). On the other hand, incidence of postoperative complications other than pulmonary complications was similar between the two groups. The authors further described that in the MIE group, the operative time of the thoracic phase was longer and blood loss was less compared with the OE group. These differences between the two groups might be associated not only with the use of thoracoscopy or laparoscopy, but also with the patient’s position and artificial pneumothorax. In the study, the position of the patients was left lateral decubitus in the OE group and prone in MIE group. Carbon dioxide (CO2) pneumothorax without selective blocking of the right lung was employed only in the MIE group. The effects of gravity and CO2 pneumothorax enable a wide surgical space. During prone position, blood pooling does not obscure the operative field and the middle mediastinal organs and right lung are naturally shifted downwards. Therefore, CO2 pneumothorax and the effects of gravity allow surgeons to visualize a dry and wide surgical space without requiring special assistants. Direct retraction of the right lung is not necessary during MIE in the prone position, thereby, avoiding mechanical lung damage and decreasing the production of inflammatory mediators.
The prone position is well known to have beneficial effects on arterial oxygenation (16). Several mechanisms have been suggested to explain the improvement in gas exchange while in a prone position. Changing from a supine position to a prone position redistributes blood flow in the lungs and makes pulmonary perfusion becomes more uniformly distributed. Furthermore, the prone position improves the diaphragmatic movement and increases functional residual capacity. A decubitus position puts pressure from the mediastinum to the ventilated lung, which may increase the risk of atelectasis (17). On the other hand, almost none of the lung tissue is located beneath the heart when a patient is in a prone position. Gravity moves the bronchial secretions and pulmonary extravascular fluid from the dorsal to the ventral side while the patient is in a prone position; this may enable the opening of bronchi that have been obstructed by secretions. As the authors performed, some investigators have been able to perform MIE in the prone position without the use of one-lung ventilation. The use of two-lung ventilation may reduce respiratory-related complications. Therefore, the low incidence of pulmonary complications after MIE can be explained by the reduction of atelectasis. The TIME trial successfully confirmed the theoretical advantages of MIE in the prone position compared with those of OE, as suggested by previous non-randomized studies (Table 1).
Table 1
| Potential advantages | Potential disadvantages |
|---|---|
| Excellent surgical space | Difficulty in emergent open thoracotomy |
| Experienced assistant not necessary needed | |
| Theoretical improved arterial oxygenation | |
| One-lung ventilation not necessary required | |
| Ergonomic position of surgical hands |
MIE, minimally invasive esophagectomy; OE, open esophagectomy.
MIE is now considered to be one of the key factors for enhanced recovery after surgery that can help reduce postoperative pain and improve recovery after esophagectomy. In the study, the authors demonstrated better QOL in terms of not only postoperative pain, but also the physical, emotional, and social components. The authors have managed both groups of patients under the same protocol during the pre-, intra-, and postoperative periods. Therefore, these results on QOL scores were mainly accounted for by the differences in surgical procedures. Although MIE has the benefit of reducing postoperative pain, the observed differences in the factors other than postoperative pain, were difficult to explain. Recently, Sun et al. conducted a single-center, open-labeled, randomized controlled trial and reported the benefit of MIE and early oral feeding to enhance postoperative management (18). In that study, the results of QOL scores were similar to those of the TIME trial; specifically, higher QOL scores on pain, physical, emotional, and social factors after MIE were obtained only in the early oral feeding group. MIE may have psychological advantages and promote a short-term postoperative course. So, far, the exact mechanism underlying the association between MIE and QOL has not yet been elucidated. Aside from the small skin incision, some other benefits, such as less invasiveness of the procedures and improvement of immunological functions, may contribute to better QOL after MIE in the prone position (Figure 1).
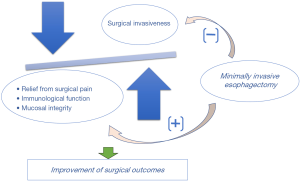
MIE has the possible advantages of preserving a patient’s immunological reactions; therefore, many investigators have attempted to reveal the surgical invasiveness and the objective parameter that reflects the less invasiveness of MIE. C-reactive protein (CRP) is a clinical parameter that represents systemic inflammatory response. Previous studies have revealed that preoperative or postoperative serum CRP levels were significantly associated with the survival rate of esophageal cancer patients (19). In our recent unpublished study, the serum CRP level on postoperative day 1 was significantly lower after MIE under prone position with CO2 pneumothorax than after MIE on the left lateral decubitus position with mini-thoracotomy. Given that precise surgical procedures without direct retraction of the right lung, mechanical lung damage can be avoided and production of inflammatory mediators can be reduced during MIE in the prone position with CO2 pneumothorax. In a future study, assessment of inflammatory cytokines, such as IL-1 and IL-6, may be required to precisely evaluate the less invasiveness of MIE.
In this follow-up study of the TIME trial, the three-year overall and disease-free survival rates were similar between OE and MIE, even after adjustments for stage of disease, gender, and age. Several randomized controlled trials have shown favorable short- and long-term outcomes of minimally invasive approaches for other gastrointestinal cancers (20). For esophageal cancer, a prospective study that evaluates the oncological safety and long-term outcome after MIE is lacking. Although there were several meta-analyses on improved short-term outcomes after MIE, to our best knowledge, this was the first prospective and randomized controlled study to demonstrate relatively prolonged oncological outcomes. Three-year survival outcomes, together with short-term outcomes, might support the use of MIE as an oncologically and technically safe surgical procedure for esophageal cancer. However, several years after esophagectomy, some patients can die from other diseases, including pneumonia. Furthermore, in the study, R0 resection of the tumor could not be performed in 14 patients (more than 12%). Considering these issues, complete results on five-year overall follow-up and analyses of the cause of deaths using a large number of patients should be recommended to clarify true benefits of MIE.
The findings of the study by Straatman et al. posed several issues that require further investigation. First, the authors employed the prone position and CO2 pneumothorax in the MIE group in this study. At present, MIE can be performed in the prone position or left lateral decubitus position. Since the report of Palanivelu et al., prone position has become a popular approach for MIE (21). However, the procedural approach to MIE varies among surgeons and institutions worldwide. Moreover, the usefulness of CO2 pneumothorax itself has not been elucidated when performing MIE. The efficacy and oncologic outcomes of MIE in the prone position with CO2 pneumothorax need to be further assessed in comparison with those of MIE in the left lateral decubitus position. Second, despite the restrictions in the study participation, MIE was converted to open thoracotomy in 6 patients (10%). This conversion rate was relatively higher compared with the 0% to 5% conversion rate in previous studies (11). These findings suggested that inclusion of surgeons with more experience on MIE and hospitals with more surgical cases of esophageal cancer might be necessary for this kind of trial. In our ongoing Japanese MIE trial, only surgeons who have experienced more than 30 OE procedures and who were credentialed by the study chair after judging their MIE skills on videos can participate in the trial (22). Furthermore, a quality assurance committee of surgery performs a central peer review of the surgical procedure for all cases using intraoperative photos. In general, the skills required to perform MIE can be difficult to master; in fact, previous investigators demonstrated a steep learning curve after dozens of MIE have been performed (23,24). Third, the ergonomics of surgeons during MIE has to be considered because esophagectomy is a technically meticulous procedure that is associated with heavy physical demands on surgeons, especially when the procedure is performed thoracoscopically. During MIE in the prone position, surgeons can operate in a plane parallel to the camera and the ports used by the operator are located at the elbow level of the surgeon; therefore, the ergonomics and fatigue experienced by the surgeons may be improved. The excellent operative view, increased magnification, and improvement of the surgeon’s ergonomics can improve the quality of mediastinal lymphadenectomy. In addition, some surgeons have emphasized that MIE in the prone position can enable precise dissection of the lymph nodes along the recurrent laryngeal nerves and those in the aortopulmonary window.
In conclusion, this follow-up study of the TIME trial, which initially presented the short-term benefits of MIE, revealed non-inferiority of MIE over OE in terms of three-year survival. These findings supported the use of MIE for the treatment of esophageal cancer. However, there is a lack of scientific evidence and objective mechanism that can represent the efficacy of MIE. Accordingly, there is a clinical need for studies that evaluate the five-year outcomes of MIE and those that develop surrogate markers that would indicate the less invasiveness of MIE using a large number of patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Shanghai Chest. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.10.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Gossot D, Cattan P, Fritsch S, et al. Can the morbidity of esophagectomy be reduced by the thoracoscopic approach? Surg Endosc 1995;9:1113-5. [Crossref] [PubMed]
- Law S, Fok M, Chu KM, et al. Thoracoscopic esophagectomy for esophageal cancer. Surgery 1997;122:8-14. [Crossref] [PubMed]
- Osugi H, Takemura M, Higashino M, et al. A comparison of video-assisted thoracoscopic oesophagectomy and radical lymph node dissection for squamous cell cancer of the oesophagus with open operation. Br J Surg 2003;90:108-13. [Crossref] [PubMed]
- Verhage RJ, Hazebroek EJ, Boone J, et al. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: a systematic review of the literature. Minerva Chir 2009;64:135-46. [PubMed]
- Nagpal K, Ahmed K, Vats A, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 2010;24:1621-9. [Crossref] [PubMed]
- Mamidanna R, Bottle A, Aylin P, et al. Short-term outcomes following open versus minimally invasive esophagectomy for cancer in England: A population-based national study. Ann Surg 2012;255:197-203. [Crossref] [PubMed]
- Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese Nationwide Web-Based Database. Ann Surg 2014;260:259-66. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally invasive versus open esophageal resection—three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomized controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Koyanagi K, Ozawa S, Tachimori Y. Minimally invasive esophagectomy performed with the patient in a prone position: a systematic review. Surg Today 2016;46:275-84. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Franchetti Y, et al. Minimally invasive esophagectomy: Results of a prospective phase II multicenter trial–the Eastern Cooperative Oncology Group (E2002) study. Ann Surg 2015;261:702-7. [Crossref] [PubMed]
- Avery KN, Metcalfe C, Berrisford R, et al. The feasibility of a randomized controlled trial of esophagectomy for esophageal cancer–the ROMIO (randomized oesophagectomy: minimally invasive or open) study: protocol for a randomized controlled trial. Trials 2014;15:200. [Crossref] [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. New Engl J Med 2002;346:1128-37. [Crossref] [PubMed]
- Maas KW, Cuesta MA, van Berge Henegouwen MI, et al. Quality of life and late complications after minimally invasive compared to open esophagectomy: results of a randomized trial. World J Surg 2015;39:1986-93. [Crossref] [PubMed]
- Bryan AC. Comments of devil’s advocate Am Rev Respir Dis 1974;110:143-4. (editorial). [PubMed]
- Yatabe T, Kitagawa H, Yamashita K, et al. Better postoperative oxygenation in thoracoscopic esophagectomy in prone positioning. J Anesth 2010;24:803-6. [Crossref] [PubMed]
- Sun HB, Li Y, Liu XB, et al. Early oral feeding following McKeown minimally invasive esophagectomy: An open-label, randomized, controlled, noninferiority trial. Ann Surg 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Matsuda S, Takeuchi H, Kawakubo H, et al. Correlation between intense postoperative inflammatory response and survival of esophageal cancer patients who underwent transthoracic esophagectomy. Ann Surg Oncol 2015;22:4453-60. [Crossref] [PubMed]
- Kitano S, Inomata M, Mizusawa J, et al. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): a phase 3, randomized controlled trial. Lancet Gastroenterol Hepatol 2017;2:261-8. [Crossref] [PubMed]
- Palanivelu C, Prakash A, Senthilkumar R, et al. Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position-experience of 130 patients. J Am Coll Surg 2006;203:7-16. [Crossref] [PubMed]
- Kataoka K, Takeuchi H, Mizusawa J, et al. A randomized phase III trial of thoracoscopic versus open esophagectomy for thoracic esophageal cancer: Japan Clinical Oncology Group study JCOG 1409. Jpn J Clin Oncol 2016;46:174-7. [Crossref] [PubMed]
- Osugi H, Takemura M, Higashino M, et al. Learning curve of video-assisted thoracoscopic esophagectomy and extensive lymphadenectomy for squamous cell cancer of the thoracic esophagus and results. Surg Endosc 2003;17:515-9. [Crossref] [PubMed]
- Song SY, Na KJ, Oh SG, et al. Learning curves of minimally invasive esophageal cancer surgery. Eur J Cardiothorac Surg 2009;35:689-93. [Crossref] [PubMed]
Cite this article as: Koyanagi K, Ozawa S. Randomized controlled trial on minimally invasive versus open esophagectomy for esophageal cancer: short and long-term outcomes. Shanghai Chest 2017;1:49.


