
Up-to date role of interventional pulmonology in the diagnosis and staging of non-small-cell lung cancer
Introduction
Lung cancer is still the leading cause of cancer deaths in both men and women worldwide, but the last decade has seen many advances in diagnosis and management options. Low-dose CT screening will probably transform the approach to lung cancer detection in the near future. Improvements in surgical techniques, introduction of new targeted therapies for individual gene mutations in non-squamous non-small cell lung cancer (NSCLC), and refinement of stereotactic radiotherapy for early stage cancers are all contributing to improve the of quality of life and the outcomes of patients. In this scenario, it is vital that the interventional pulmonologist integrates the armamentarium of minimally invasive techniques described in this review to provide all the specialists of the lung cancer multidisciplinary team with the information needed for optimal management of each specific case. The present review is meant to provide a systematic, yet concise evaluation of the role of interventional pulmonology procedures in each of the descriptors of the TNM (Tumor, Node, Metastasis) staging system of lung cancer.
“T” descriptor
In the assessment of the “T” descriptor for diagnostic purposes, the first key moment for the interventional pulmonologist is the review and the correct interpretation of the imaging data aimed at selecting the sampling procedure with the best possible balance between diagnostic success and complication rate.
Although a lung primary tumor may basically have either a central or a peripheral location, significantly different consideration may apply to individual lesions belonging to each of the above categories when it comes to choose how to biopsy them, as more specifically described below.
Central lesions
A central primary tumor is defined as a lesion that is visible at bronchoscopy and/or is located in the inner third of the lung parenchyma (1). For practical purposes, a central lesion may grow with one the following three patterns, and each of them requires a different diagnostic approach by the interventional pulmonologist: exophytic/infiltrating pattern (Figure 1), peribronchial pattern with airway compression (Figure 2), peribronchial pattern with no airway compression (Figure 3).
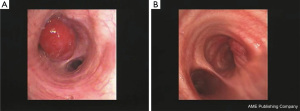
In the exophytic/infiltrating pattern, the bronchial mucosa is involved by the tumor and simple forceps biopsy, when correctly carried out, allows a definite diagnosis to be achieved in the vast majority of cases. When the surface of the lesion looks whitish due to possibly extensive necrosis, the use of a needle (transbronchial needle aspiration) that by-passes the surface and samples the lesions more in depth may reduce the risk of not getting viable tissue.
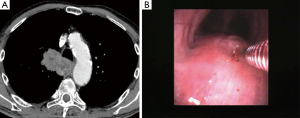
In the peribronchial pattern with airway compression, the bronchial mucosa is intact and forceps biopsy would not help you get the diagnosis. In these cases, using a needle that pierces the normal mucosa and reaches the lesion growing beyond the airway wall is indicated. Although the availability of endobronchial ultrasound (EBUS) guidance allows to elegantly visualize the lesion in almost every case and sample it safely in real time, a blind transbronchial needle aspiration procedure can also be carried out successfully by using the landmark represented by the area of airway compression, especially when this area is quite large and evident (Figure 2).
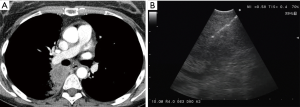
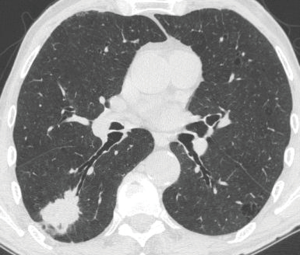
In the peribronchial pattern with no airway compression, the lesion is totally invisible at bronchoscopy, regardless of its size, as is grows beyond the airway and does not compress it. In this specific case, optimization of diagnostic yield and reduction of possible complications can only be achieved by using the ultrasound guidance (Figure 3). Literature suggests that linear EBUS has a very high diagnostic yield (>90%) and a negligible complication rate when used to sample central parenchymal lesions not visible at standard bronchoscopy (2-4). Less frequently, the primary tumor can be difficult or impossible to access from the airways, but adjacent to the esophagus; in these cases, transesophageal needle aspiration with ultrasound guidance using an echobronchoscope (EUS-b-FNA) or a regular echogastroscope (EUS-NA) may allow to successfully sample the tumor (Figure 4) (5).
Peripheral lesions
Peripheral tumors are located in the outer third of the lung and are not visible at standard bronchoscopy. The diagnostic yield of bronchoscopy for diagnosis of peripheral nodules is influenced by a variety of factors (Table 1).
Table 1
| Lesion size |
| Bronchus sign |
| Sampling procedure (forceps biopsy, TBNA, brushing, washing) |
| Operator skills/experience |
| Nature of lesion (malignant vs. benign) |
| Number of specimens taken |
| Imaging guide: |
| Virtual bronchoscopy |
| Electromagnetic navigation bronchoscopy |
| Radial endobronchial ultrasound |
| Fluoroscopy |
| CT-fluoroscopy |
| Ultrathin bronchoscopy |
In the past decade, the technological progress has led to the introduction in the market of several useful guidance systems, such as radial EBUS, electromagnetic navigation, and virtual bronchoscopy navigation that may help successfully steer the bronchoscopic sampling devices (forceps, needles, brushes) towards peripheral pulmonary nodules for accurate biopsy. Individual studies and systematic reviews regarding guided bronchoscopy with newly introduced systems suggest that a diagnostic yield of approximately 70% can be steadily reached with a very low complication rate (6-12). The drawbacks of these new methods are their quite high initial and running costs, as well as their time-consume, making them more easily doable under moderate or deep sedation.
Besides guidance systems, other factors may significantly influence the success rate of bronchoscopy in the setting of peripheral lesions (Table 1). While larger nodule size (>2 cm) and greater operator skills/experience intuitively increase the success rate (12), the influence of the presence/absence of the bronchus sign as well as the sampling method that is used for biopsy are often undervalued.
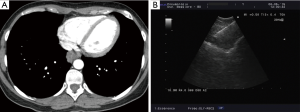
The presence of the so called “bronchus sign”, a radiologic sign visible at CT and characterized by the presence of a bronchus leading to the lesion (Figure 5), is a key factor predicting a successful biopsy regardless of the guidance system that is used (13-15).
As for the device used for sampling, evidence from the literature suggests that transbronchial needle aspiration is the single method associated with the best diagnostic success, and should always be used, along with transbronchial lung biopsy, to optimize the diagnostic yield of bronchoscopy. It is thought that most of the added value of TBNA in patients with peripheral nodules or masses is related to the ability to diagnose peribronchial lesions. Unlike other bronchoscopic sampling tools, such as forceps for TBLB, which follow the airway lumen and are particularly valuable in the presence of lesions that grow, at least partially, within the lumen (intraluminal lesions), TBNA can be used to pierce the bronchus and also reach peribronchial lesions. Interestingly, the superiority of TBNA versus TBLB in the approach to peripheral lesions is independent of the guidance system used to reach the lesion (12,16,17).
“N” descriptor
The possibility to sample hilar and mediastinal lesions located besides the airway wall has been certainly the greatest advance associated with the use of flexible bronchoscopy. Reports on the attempt to sample mediastinal lymph nodes endoscopically date back to the 40s when E. Schieppati described the first transbronchial needle aspiration, performed during rigid bronchoscopy on a subcarinal node (18,19), but the procedure did not actually gain large popularity and widespread use probably due to technical difficulties associated with the use of rigid bronchoscopy (discomfort to the patient or need for general anesthesia, and lack of training in rigid bronchoscopy for respiratory physicians), and the contemporary development of more definitive surgical procedures such as mediastinoscopy.
The father of modern TBNA is KP Wang who, without knowledge of the abovementioned experiences, performed in 1978, five right paratracheal lymph node aspirations during rigid bronchoscopy with an esophageal varices needle (20). Encouraged by the results of the procedure and by the absence of remarkable bleeding, Wang predicted the potential application of TBNA with the flexible bronchoscope and focused on the development of flexible, disposable needles. The first dedicated transbronchial aspiration needle for use with flexible bronchoscope became available in 1983 (10), and was subsequently modified to provide better protection of the scope (21) and better suction capacity (22). The first histology needle for use with flexible bronchoscopy was developed in 1985 (23) and was subsequently improved to overcome the same problems experienced with the needle for cytology specimens (24). Almost 40 years after its introduction, strengths and limits of conventional TBNA are well known and have been listed in a recent systematic review (25). Major predictors of success in an unselected population, as well as in patients with suspected/known lung cancer, include lymph node size (short axis length ≥2 cm), presence of abnormal endoscopic findings, subcarinal and right paratracheal location, and the use of histological needle by an experienced bronchoscopist (25). A very interesting meta-analysis on the results of TBNA in the mediastinal staging of lung cancer selected, based on rigorous criteria, 13 studies out of 525 initially taken into account (26). Such analysis basically confirmed the very high specificity of the method, but also clearly showed that its sensitivity largely depends on the prevalence of lymph node metastasis in the population being studied. In particular, the sensitivity of TBNA proved high in studies with high prevalence of N2–N3 involvement, and the general implication was that the mediastinal nodes were markedly enlarged in these study populations. On the contrary, TBNA yield was much lower than previously thought in populations with low prevalence of lymph node metastasis. These data suggest that the primary role for TBNA in the mediastinal staging of NSCLC should be that of confirming a neoplastic lymph node involvement which looks likely based on the results of imaging techniques, by virtue of its high specificity and sensitivity in this specific setting (26). The main reason for the above findings is that conventional TBNA is essentially a “blind” procedure, as target lymph nodes cannot be visualized directly by the operator and the site for aspiration/biopsy is chosen based on the knowledge of a few endobronchial landmarks and prior contrast enhancement CT evaluation. In the last 15 years, the advent of endobronchial ultrasounds guided transbronchial needle aspiration (EBUS-TBNA), a technique that couples ultrasounds and TBNA has led to a significant improvement of the success rate of bronchoscopic mediastinal sampling and has markedly reduced the need for diagnostic and staging surgical procedures, such as mediastinoscopy and video-assisted thoracoscopy (VATS). EBUS-TBNA is performed using a dedicated bronchoscope with a curvilinear ultrasound transducer located at its tip, and has a dedicated working channel that allows the introduction of dedicated needles. EBUS-TBNA is, by now, state of art in mediastinal diagnosis, mediastinal staging and mediastinal restaging of lung cancer owing to its simplicity, safety and effectiveness.
Mediastinal diagnosis
EBUS-TBNA is often the easiest way to retrieve diagnostic tissue in patients with suspected lung cancer, and it represents the first step diagnostic technique in many patients with advanced lung cancer, especially if the lung primary tumor is peripheral (Figure 6). When used in this specific setting, the aim of the procedure is not only histologic diagnosis and accurate phenotyping, but also genotyping.
A number of individual studies suggest that the diagnostic success of EBUS-TBNA in the mediastinal diagnosis of lung cancer exceeds 85–95%, and that the procedure is extremely safe (27-34).
Interestingly, EBUS-derived samples have proven amenable for extensive cancer genotyping, a condition which is key to its continued success in the era of personalized treatment of lung cancer. While testing for a limited number of genes (EGFR, KRAS, and ALK) on EBUS-samples has been proven successful in the vast majority (>90%) of patients (35-39), new molecular markers with important therapeutic implications (i.e., ROS1, PDL1) require testing for first-line oncological treatment, and many more will probably come in the near future (40).
In the attempt to keep the pace of this evolution, several steps are being implemented. First, international associations such as the American College of Chest Physicians and the World Association for Bronchology and Interventional Pulmonology have reviewed the pertinent literature and have issued guidelines on the acquisition and processing of EBUS-TBNA samples with the idea of optimizing and possibly standardizing it, as well as promoting research in the gray areas (41,42). Meanwhile, the industry is trying to help the clinicians get better material in terms of quality and quantity by producing new needles such as the pro-core needles or the 19-gauge needles (43-47). Finally, improvement in the sensitivity of the tools used for testing (i.e., Next Generation Sequencing) will probably provide the chance to test more genes with a limited amount of neoplastic tissue (48-51).
Mediastinal staging
Accurate mediastinal staging of lung cancer is key to appropriate treatment planning in the absence of distant metastases. Endosonography (EBUS-TBNA ± EUS-NA/EUS-b-FNA) represents a valid alternative to surgical staging, as shown by studies demonstrating improved nodal tissue staging, reduction of number of futile thoracotomies, and cost-effectiveness (34,52-55). In the most robust of such trials, patients undergoing mediastinal staging were randomized towards mediastinoscopy or endosonography (EBUS + EUS), followed by mediastinoscopy in those cases in which no metastases were found by endosonography (53). The investigators found a 79% sensitivity for endosonography, an 85% sensitivity for mediastinoscopy and a 94% sensitivity for endosonography followed by mediastinoscopy (53). Based on these results, both the ACCP and the combined ESGE/ERS/ESTS guidelines suggest that endosonography, followed by mediastinoscopy in patients with negative endosonography but high risk of mediastinal metastasis, should be the preferred staging modality (56-58).
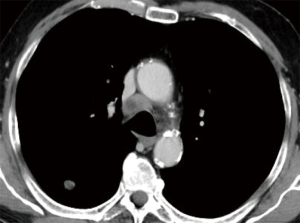
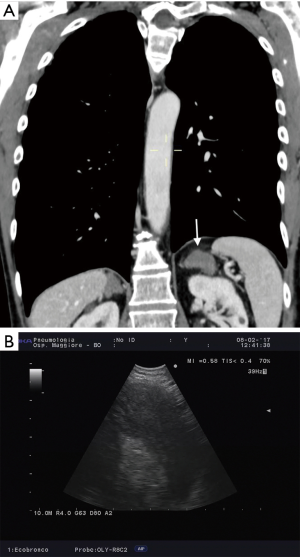
The importance of surgical staging after a negative endosonography evaluation remains particularly important in lung cancer patients lacking evidence of lymph node metastases at imaging studies (CT and PET) if the tumor is centrally located (Figure 7) or has clinical N1 involvement (Figure 8). The prevalence of occult mediastinal metastases (i.e., as metastases detected at surgery in spite of negative CT and PET) in these patients, in fact, may be as high as 20–25%, whereas the results of mediastinal staging via endosonography may be variable and somehow unsatisfactory (Table 2).
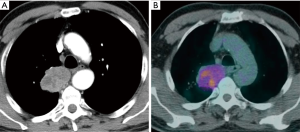
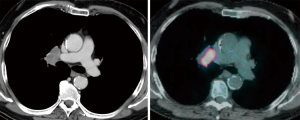
Table 2
| Study | Design | Patients | Region | Inclusion criteria | Index test | Ref. standard | Prevalence of N2 | Sensitivity/NPV |
|---|---|---|---|---|---|---|---|---|
| Dooms et al. Chest 2015 | Prospective, multicenter | 100 | EU | Resectable pts with cN1 (CT-PET) | EBUS ± EUS | Surgery | N2 = 24% | 38%/83% |
| Shingyoji et al. ATS 2014 | Retrospective, single center | 113 | Japan | Resectable pts with cN0 (CT-PET) | EBUS | Surgery | N2 = 17.6% | 35%/88% |
| Ong et al. Ann ATS 2015 | Retrospective, multicenter | 220 | USA | Resectable with cN0 (CT-PET) | EBUS | Surgery or imaging follow-up | N1 + N2 = 22%; N2 + N3 = 8% | 36.7%/84.7% |
Initial studies that evaluated the staging role of EBUS in lung cancer patients with either radiologically (lymph nodes <1 cm at CT) or PET normal mediastinum attributed at this method sensitivity and negative predictive values (NPV) as high as 92%/96% and 89%/99%, respectively (59,60). More recent trials performed in the same setting came to quite different results. Shingyoji et al. carried out a single center retrospective analysis of their 113 EBUS procedures performed for staging purposes in lung cancer patients with negative mediastinum at CT and PET. The prevalence of occult mediastinal metastases in this series was 17.6%, whereas sensitivity and NPV of EBUS were 38% and 88%, respectively (61). Ong et al. retrospectively review the EBUS-TBNAs performed for lung cancer staging at two major academic centers from 2009 to 2014 (62). Two hundred and twenty patients with radiographic N0 disease [lymph nodes (LN) ≤1 cm in the short axis and SUV max ≤2.5 by PET/CT] were included. Primary outcome was sensitivity and NPV of EBUS-TBNA. Overall sensitivity and NPV of EBUS were 36% and 85%, respectively. Excluding patients with occult disease “outside” the reach of EBUS the sensitivity and NPV of EBUS were 60% and 93%, respectively.
Dooms et al. performed a prospective multicentric study aimed at evaluating the role of endosonography in patients with clinical N1 tumors (63). Consecutive patients with operable and resectable cN1 NSCLC underwent a lobe specific mediastinal nodal staging by endosonography. The primary study outcome was sensitivity to detect N2 disease. The secondary end points were the prevalence of N2 disease, the NPV of both endosonography and endosonography with confirmatory mediastinoscopy, and the number of patients needed to detect one additional N2 disease with mediastinoscopy. Of the 100 patients with cN1 on imaging, 24 patients were diagnosed with N2 disease. Invasive mediastinal nodal staging with endosonography alone had a sensitivity of 38%, which increased to 73% by adding a mediastinoscopy. NPV was 81% and 91%, respectively. Ten mediastinoscopies were needed to detect one additional N2 disease missed by endosonography.
Although the latter 3 studies have been criticized because the use of EUS along with EBUS was not used in 2 (61-63) and was left at the discretion of the each investigators in the remaining one (63), the suboptimal results of EBUS staging in lung cancer patients with normal mediastinum at imaging studies or cN1 deserve further attention and, possibly, evaluation in larger prospective well designed trials.
Mediastinal re-staging
The role of endosonography for mediastinal restaging of N2 disease after induction chemotherapy and/or radiotherapy has been evaluated in a limited number of studies with a similar study design (Table 3) (64-68). Albeit variable, the sensitivity of endosonography in this setting (50–82%) proved somewhat lower than that usually achieved in lung cancer patients undergoing the procedure for upfront mediastinal staging. Furthermore, data show a quite evident relationship between prevalence of residual N2 disease after induction treatment and the sensitivity of EBUS restaging (Table 3). Interestingly, in the only study in which restaging was carried out with combined EBUS and EUS, the overall sensitivity (67%) was significantly higher than that of either EBUS (48%, P<0.001) or EUS (61%, P<0.02) alone (67). Small lymph node size, presence of extensive lymph node fibrosis, very limited metastatic lymph node involvement, and metastatic involvement of lymph nodes out of reach of EBUS (i.e., station #6) are the main reasons taken into account to explain the suboptimal result of mediastinal restaging with endosonography (64-68).
Table 3
| Study | Design | Patients | Region | Inclusion criteria | Index test | Ref. standard | Prevalence of N2 | Sensitivity/NPV |
|---|---|---|---|---|---|---|---|---|
| Herth et al. JCO 2008 | Retrospective, multicenter | 124 | EU, USA, Japan | N2 disease after neoadjuvant CT | EBUS | Surgery | 94% | 76%/20% |
| Szlubovski et al. EJCTS 2010 | Prospective, single center | 61 | Poland | N2 disease after neoadjuvant CT | EBUS | Surgery | 44% | 67%/78% |
| Szlubovski et al. EJCTS 2014 | Prospective, single center | 106 | Poland | N2 disease after neoadjuvant CT | EBUS + EUS-b | Surgery | 51.90% | 67%/73% |
| Nasir et al. ATS 2014 | Retrospective, single center | 32 | USA | N2 disease after neoadjuvant CT | EBUS or EUS | Surgery or imaging follow-up | 18.80% | 50%/88% |
| Cetinkaya et al. Endosc Ultras 2017 | Retrospective, multicenter | 44 | Turkey | N2 disease after neoadjuvant CT and/or RT | EBUS | Surgery | 63.60% | 82%/88% |
Given the above results, endosonography for mediastinal restaging can be considered a useful test to confirm, but not to rule out metastatic lymph node involvement. Therefore, surgical staging remains crucial in patients with negative endosonography results (64-68).
“M” descriptor
The interventional pulmonologist may also have a role in confirming confirm the presence of intra and extrathoracic metastases belonging to the “M” descriptor.
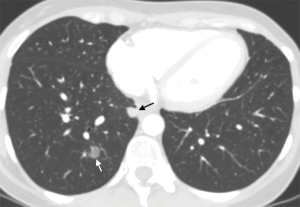
It is increasingly more common to see patients with multiple lung nodules, sometimes bilateral, that can be either expression of metastatic disease or of multiple synchronous lesions. Use of the previously described endoscopic biopsy procedures aimed at sampling parenchymal lesions, coupled with molecular biology testing aimed at studying the genetic status of the different lesions may help classify them correctly and help choose the most appropriate treatment plan (Figure 9) (69).
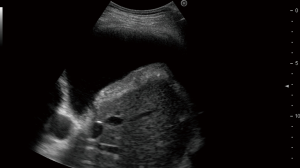
Metastatic pleural effusion can be safely and elegantly demonstrated with ultrasound-assisted thoracocentesis (Figure 10) or medical thoracoscopy (70).
Furthermore, recent data demonstrate that the left adrenal can be seen and safely sampled transgastrically by the interventional pulmonologist with a regular EUS in the vast majority of patients (Figure 11). In a pilot study, Crombag et al. could identify the left adrenal in 85% of patients in whom an attempt was made to visualize the gland (71). More recently, a multicenter randomized trial showed that left adrenal gland could be analyzed and sampled with similar success rate with the regular echogastroscope as well as with an EUS (72).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.10.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Casal RF, Vial MR, Miller R, et al. What Exactly Is a Centrally Located Lung Tumor? Results of an Online Survey. Ann Am Thorac Soc 2017;14:118-23. [Crossref] [PubMed]
- Tournoy KG, Rintoul RC, van Meerbeeck JP, et al. EBUS-TBNA for the diagnosis of central parenchymal lung lesions not visible at routine bronchoscopy. Lung Cancer 2009;63:45-9. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Fujiwara T, et al. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for the Diagnosis of Intrapulmonary Lesions. J Thorac Oncol 2008;3:985-8. [Crossref] [PubMed]
- Verma A, Jeon K, Koh WJ, et al. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for the Diagnosis of Central Lung Parenchymal Lesions. Yonsei Med J 2013;54:672-8. [Crossref] [PubMed]
- Korevaar DA, Colella S, Spijker R, et al. Esophageal Endosonography for the Diagnosis of Intrapulmonary Tumors: A Systematic Review and Meta-Analysis. Respiration 2017;93:126-37. [Crossref] [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [Crossref] [PubMed]
- Ali MS, Trick W, Mba BI, et al. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Respirology 2017;22:443-53. [Crossref] [PubMed]
- Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011;37:902-10. [Crossref] [PubMed]
- Gex G, Pralong JA, Combescure C, et al. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration 2014;87:165-76. [Crossref] [PubMed]
- Zhang W, Chen S, Dong X, et al. Meta-analysis of the diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules. J Thorac Dis 2015;7:799-809. [PubMed]
- Asano F, Eberhardt R, Herth FJ. Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respiration 2014;88:430-40. [Crossref] [PubMed]
- Rivera MP, Mehta AC, Wahidi MM. Establishing the Diagnosis of Lung Cancer. Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013;143:e142S-65S.
- Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a Bronchus sign on CT imaging: results from a prospective study. Chest 2010;138:1316-21. [Crossref] [PubMed]
- Guvenc C, Yserbyt J, Testelmans D, et al. Computed tomography characteristics predictive for radial EBUS-miniprobe-guided diagnosis of pulmonary lesions. J Thorac Oncol 2015;10:472-8. [Crossref] [PubMed]
- Bilaçeroğlu S, Kumcuoğlu Z, Alper H, et al. CT bronchus sign-guided bronchoscopic multiple diagnostic procedures in carcinomatous solitary pulmonary nodules and masses. Respiration 1998;65:49-55. [Crossref] [PubMed]
- Chao TY, Chien MT, Lie CH, et al. Endobronchial ultrasonography-guided transbronchial needle aspiration increases the diagnostic yield of peripheral pulmonary lesions. A randomized trial. Chest 2009;136:229-36. [Crossref] [PubMed]
- Trisolini R, Cancellieri A, Tinelli A, et al. Performance characteristics and predictors of yield from transbronchial needle aspiration in the diagnosis of peripheral pulmonary lesions. Respirology 2011;16:1144-9. [Crossref] [PubMed]
- Schieppati E. La punction mediastinal a traves del espolon traqueal. Review of the Argentine Medical Association 1949;663:497.
- Schieppati E. Mediastinal lymph node puncture through the tracheal carina. Surg Gynecol Obstet 1958;107:243-6. [PubMed]
- Wang KP, Terry P, Marsch B. Bronchoscopic needle aspiration in the diagnosis and staging of bronchogenic carcinoma. Chest 1983;84:571-6. [Crossref] [PubMed]
- Gittlen SD, Erozan Y, Wang KP. A new versatile transbronchial cytology needle for the staging and diagnosis of bronchogenic carcinoma. Chest 1988;94:561-5. [Crossref] [PubMed]
- Wang KP, Selcuk ZT, Erozan Y. Transbronchial needle aspiration for cytology specimens. Monaldi Arch Chest Dis 1994;49:265-7. [PubMed]
- Wang KP. Flexible transbronchial needle aspiration biopsy for histologic specimens. Chest 1985;88:860-3. [Crossref] [PubMed]
- Wang KP. Transbronchial needle aspiration to obtain histology specimen. J Bronchol 1994;1:116-22. [Crossref]
- Bonifazi M, Zuccatosta L, Trisolini R, et al. Transbronchial needle aspiration: a systematic review on predictors of a successful aspirate. Respiration 2013;86:123-34. [Crossref] [PubMed]
- Holty JE, Kushner WG, Gould MK. Accuracy of transbronchial needle aspiration for mediastinal staging of non-smal cell lung cancer: a meta-analysis. Thorax 2005;60:949-55. [Crossref] [PubMed]
- Krasnik M, Vilmann P, Larsen SS, et al. Preliminary experience with a new method of endoscopic transbronchial real-time ultrasound guided biopsy for diagnosis of mediastinal and hilar lesions. Thorax 2003;58:1083-6. [Crossref] [PubMed]
- Rintoul RC, Skwarski KM, Murchinson JT, et al. Endobronchial and endoscopic ultrasound-guided real-time fine needle aspiration for mediastinal staging. Eur Respir J 2005;25:416-21. [Crossref] [PubMed]
- Vilmann P, Krasnik M, Larsen SS, et al. Transesophageal endoscopic ultrasound-guided fine-needle aspiration and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy: a combined approach in the evaluation of mediastinal lesions. Endoscopy 2005;25:833-9. [Crossref] [PubMed]
- Yasufuku K, Chiyo M, Koh E, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer 2005;50:347-54. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Vilmann P, et al. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph-nodes. Thorax 2006;61:795-8. [Crossref] [PubMed]
- Yasufuku K, Nakajima T, Motoori K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest 2006;130:710-8. [Crossref] [PubMed]
- Bauwens O, Dusart M, Pierard P, et al. Endobronchial ultrasound and value of PET for prediction of pathological results of mediastinal hot spots in lung cancer patients. Lung Cancer 2008;61:356-61. [Crossref] [PubMed]
- Ernst A, Anhantam D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008;3:577-82. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Nakagawara A, et al. Multigene mutation analysis of metastatic lymph nodes of non-small-cell lung cancer diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Chest 2011;140:1319-24. [Crossref] [PubMed]
- Yarmus L, Akulian J, Gilbert C, et al. Optimizing Endobronchial Ultrasound for Molecular Analysis: How many passes are needed? Ann Am Thorac Soc 2013;10:636-43. [Crossref] [PubMed]
- Folch E, Yamaguchi N, VanderLaan PA, et al. Adequacy of lymph node transbronchial needle aspirates using convex probe endobronchial ultrasound for multiple tumor genotyping techniques in non-small-cell lung cancer. J Thorac Oncol 2013;8:1438-44. [Crossref] [PubMed]
- Trisolini R, Cancellieri A, Tinelli C, et al. Randomized trial of endobronchial ultrasound-guided transbronchial needle aspiration with and without rapid on-site evaluation for lung cancer genotyping. Chest 2015;148:1430-7. [Crossref] [PubMed]
- Casadio C, Guarize J, Donghi S, et al. Molecular Testing for Targeted Therapy in Advanced Non-Small Cell Lung Cancer: Suitability of Endobronchial Ultrasound Transbronchial Needle Aspiration. Am J Clin Pathol 2015;144:629-34. [Crossref] [PubMed]
- Hiley CT, Le Quesne J, Santis G, et al. Challenges in molecular testing in non-small-cell lung cancer patients with advanced disease. Lancet 2016;388:1002-11. [Crossref] [PubMed]
- van der Heijden EH, Casal RF, Trisolini R, et al. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound-guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration 2014;88:500-17. [Crossref] [PubMed]
- Wahidi MM, Herth FJ, Yasufuku K, et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016;149:816-35. [Crossref] [PubMed]
- Gnass M, Sola J, Filarecka A, et al. Initial Polish experience of Flexible 19 gauge Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration. Adv Respir Med 2017;85:64-8. [Crossref] [PubMed]
- Tyan C, Patel P, Czarnecka Z, et al. Flexible 19-Gauge Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Needle: First Experience. Respiration 2017;94:52-7. [Crossref] [PubMed]
- Parthiban S, Dillard D, Sczaniecka A, et al. A Novel 19g EBUS-TBNA Needle: Results of Ex Vivo Tissue Evaluations. Chest 2017;150:976A. [Crossref]
- Xing J, Manos S, Monaco SE, et al. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: A Pilot Study to Evaluate the Utility of the ProCore Biopsy Needle for Lymph Node Sampling. Acta Cytol 2016;60:254-9. [Crossref] [PubMed]
- Trisolini R, Natali F, Ferrari M, L, et al. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration With The Flexible 19-Gauge Needle. Clin Resp J ; accepted for publication.
- Stoy S, Murgu S. The use of endobronchial ultrasound guided transbronchial needle aspiration specimens for next generation sequencing in non-small cell lung cancer: a clinical perspective. J Thorac Dis 2017;9:E398-401. [Crossref] [PubMed]
- Fielding D, Dalley AJ, Bashirzadeh F, et al. Next-Generation Sequencing of Endobronchial Ultrasound Transbronchial Needle Aspiration Specimens in Lung Cancer. Am J Respir Crit Care Med 2017;196:388-91. [Crossref] [PubMed]
- Leong TL, Christie M, Kranz S, et al. Evaluating the genomic yield of a single endobronchial ultrasound-guided transbronchial needle aspiration in lung cancer: meeting the challenge of doing more with less. Clin Lung Cancer 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Rooper LM, Nicolskaia O, Carter J, et al. A single EBUS-TBNA procedure can support a large panel of immunohistochemical stains, specific diagnostic subtyping, and multiple gene analyses in the majority of non-small cell lung cancer cases. Hum Pathol 2016;51:139-45. [Crossref] [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective con- trolled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for medias- tinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [Crossref] [PubMed]
- Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245-52. [Crossref] [PubMed]
- Rintoul RC, Glover MJ, Jackson C, et al. Cost effectiveness of endosonography versus surgical staging in potentially resectable lung cancer: a health economics analysis of the ASTER trial from a European perspective. Thorax 2014;69:679-81. [Crossref] [PubMed]
- Sharples LD, Jackson C, Wheaton E, et al. Clinical effectiveness and cost-effectiveness of endobronchial and endoscopic ultrasound relative to surgical staging in potentially resectable lung cancer: results from the ASTER randomised controlled trial. Health Technol Assess 2012;16:1-75. iii-iv. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-e250S.
- Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and oesophageal endosonography for the diagnosis and staging of lung cancer. European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Eur Respir J 2015;46:40-60. [Crossref] [PubMed]
- Herth FJ, Ernst A, Eberhardt R, et al. Endobronchial ultrasound guided transbronchial needle aspiration of lymph nodes in the radiologically normal mediastinum. Eur Respir J 2006;28:910-4. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Krasnik M, et al. Endobronchial ultrasound guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest 2008;133:887-91. [Crossref] [PubMed]
- Shingyoji M, Nakajima K, Yoshino M, et al. Endobronchial Ultrasonography for Positron Emission Tomography and Computed Tomography–Negative Lymph Node Staging in Non-Small Cell Lung Cancer. Ann Thorac Surg 2014;98:1762-7. [Crossref] [PubMed]
- Ong P, Grosu H, Eapen G, et al. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for Systematic Nodal Staging of Lung Cancer in Patients with N0 Disease by CT and Integrated PET/CT. Ann Am Thor Soc 2015;12:297-9. [Crossref]
- Dooms C, Tournoy KG, Shuurbiers O, et al. Endosonography for Mediastinal Nodal Staging of Clinical N1 Non-small Cell Lung Cancer. A prospective multicenter study. Chest 2015;147:209-15. [Crossref] [PubMed]
- Herth FJ, Annema J, Eberhardt R, et al. Endobronchial Ultrasound With Transbronchial Needle Aspiration for Restaging the Mediastinum in Lung Cancer. J Clin Oncol 2008;26:3346-50. [Crossref] [PubMed]
- Szlubowski A, Herth FJ, Soja J, et al. Endobronchial ultrasound-guided needle aspiration in non-small-cell lung cancer restaging verified by the transcervical bilateral extended mediastinal lymphadenectomy — a prospective study. Eur J Cardiothorac Surg 2010;37:1180-4. [Crossref] [PubMed]
- Nasir BS, Briant AS, Minnich DJ, et al. The Efficacy of Restaging Endobronchial Ultrasound in Patients With Non-Small Cell Lung Cancer After Preoperative Therapy. Ann Thorac Surg 2014;98:1008-12. [Crossref] [PubMed]
- Szlubowski A, Zielinski M, Soja J, et al. Accurate and safe mediastinal restaging by combined endobronchial and endoscopic ultrasound-guided needle aspiration performed by single ultrasound bronchoscope. Eur J Cardiothorac Surg 2014;46:262-6. [Crossref] [PubMed]
- Cetinkaya E, Usluer O, Yilmaz A, et al. Is endobronchial ultrasound-guided transbronchial needle aspiration an effective diagnostic procedure in restaging of non-small cell lung cancer patients? Endosc Ultrasound 2017;6:162-7. [Crossref] [PubMed]
- Forti Parri SN, Bonfanti B, Cancellieri A, et al. Molecular analysis driven video-assisted thoracic surgery resections in bilateral synchronous lung cancers: from the test tube to the operatory room. Ann Transl Med 2017;5:397. [Crossref]
- Havelock T, Teoh R, Laws D, et al. Pleural procedures and thoracic ultrasound: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65:ii61-76. [Crossref] [PubMed]
- Crombag LM, Annema JT. Left Adrenal Gland Analysis in Lung Cancer Patients Using the Endobronchial Ultrasound Scope: A Feasibility Trial. Respiration 2016;91:235-40. [Crossref] [PubMed]
- Crombag LM, Szlubowski A, Stigt JA, et al. EUS-B-FNA vs conventional EUS-FNA for left adrenal gland analysis in lung cancer patients. Lung Cancer 2017;108:38-44. [Crossref] [PubMed]
Cite this article as: Trisolini R, Natali F, Fois A. Up-to date role of interventional pulmonology in the diagnosis and staging of non-small-cell lung cancer. Shanghai Chest 2017;1:50.


