A perspective on lung volume reduction surgery for pulmonary emphysema
The surgical physiology of lung volume reduction (LVR)
There is strong evidence that lung volume reduction surgery (LVRS) provides both symptomatic and physiological improvement in patients with advanced emphysema (1). Emphysema is a progressive disease in which destruction of airways architecture causes a loss of alveolar elastic recoil, air trapping and resultant hyperinflation of the chest wall and diaphragm with under-ventilation, rather than compression, of the less compliant and healthier lung parenchyma. There is a detrimental effect on chest wall mechanics and the inhaled and exhaled volumes achieved during the respiratory cycle are considerably reduced. Emphysema morphology is variable with clear areas of more severe damage (heterogeneous) or with a more diffuse pattern (homogeneous) throughout the lungs (2).
LVRS aims to improve chest wall mechanics, and therefore reduce the work of breathing and the sensation of breathlessness, by excision of the most damaged emphysematous lung parenchyma, thereby reducing hyperinflation. The chest wall and diaphragm return to a more normal position, thus restoring some of the functional capacity leading to greater volumes of air movement throughout the respiratory cycle (3). The increased ventilation of the healthier, well perfused lung tissue may lead to improved ventilation/perfusion matching with a beneficial effect on right ventricular hypertrophy and pulmonary hypertension.
Patient selection
Attention to detail in patient selection is probably more important than the actual detail of surgical technique in LVRS. We continue to adopt the broad selection criteria set out in the National Emphysema Treatment Trial (NETT) a randomised study that enrolled 1,218 patients across 17 centres and showed significant benefits of LVRS compared to best medical therapy. Inclusion criteria include: obstruction (FEV1 <40% predicted); distension (RV >150% predicted with an RV:TLC >60); limited destruction DLCO >20% predicted and PCO2 <7KPa and heterogeneity with upper lobe predominant target areas. Pulmonary hypertension (MPAP >40 mmHg) is exclusion criteria. All patients should have stopped smoking for over 3 months and have completed a pulmonary rehabilitation programme for 6–10 weeks. The prepared patients for surgery both physically by building on core musculature thereby maximising muscle strength and psychologically by reducing the sensation of dyspnoea (4). Moy et al. found that patients achieved the greatest benefit from LVRS when pulmonary rehabilitation was part of an integrated perioperative programme (5). Furthermore, pulmonary rehabilitation supports postoperative recovery and reduces complications. Smoking cessation and the ability of a patient to undergo pulmonary rehabilitation are both strong indicators of compliance (5).
In our practice we follow similar selection criteria to that of the NETT trial but have derived a risk model which allows us to individualise the risk for each patient allowing for a more informed patient contribution to selection (6).
The surgical approach
Which incision?
In 1959, Dr. Brantigen (7) first described the concept of resection of the diffusely emphysematous portion of the lung to bring symptomatic relief .The pulmonary resection was carried out through a thoracotomy together with radical hilar stripping and plication of the diaphragm. Results were poor and associated with a high mortality and the procedure was not revived until 1995 when Low et al. (8) performed median sternotomy and bilateral non-anatomical resection of the most severely destroyed, functionless tissue to reduce lung volume by 20–30%. Soon the operation was offered using video assisted thoracic surgery (VATS) and there have been several non-randomized comparisons.
In NETT both approaches had similar risks of 30-day, 90-day, and overall mortality. There were no differences in complication rates, changes in exercise capacity and lung function, but patients who had undergone a VATS procedure were more likely to have a faster recovery time and generally incurred less cost (9). There have been some further conflicting findings with some showing similar outcome for operative mortality and lung function improvement (10,11) and others (12) finding a significant advantage for VATS group with respect to the in-hospital mortality rate (VATS, 2.5%, versus sternotomy, 13.8%). The current opinion is in favour of VATS as approach of choice.
One lung or two?
In NETT patients underwent bilateral, one-stage LVRS but subsequent reports have suggested the benefits of a staged unilateral approach.
Brenner et al. (10) found that there was a greater short-term physiological benefit from bilateral LVRS but noted a more rapid rate of FEV1 decline in these patients, this may be explained by an increase of the normal daily stress on alveoli during tidal breathing which in turn accelerates the age-related increase in alveolar size in the remaining lung.
We have found bilateral LVRS to be associated with an increased ventilation time, ITU and in-hospital stay whereas in terms of lung function and health status the unilateral LVRS produced results comparable to the bilateral group but also had faster recovery times with less morbidity (11). Hazelrigg et al. compared one-stage bilateral LVRS with staged LVRS—the average time between the two operations was 3 to 4 months—and he found that there was no difference in postoperative clinical improvement and complication rate and in-hospital stay (12). We have persuaded that staged bilateral LVRS provides comparable results to bilateral LVRS, with regard to lung function and health status but resulted in faster recovery with less morbidity; it gives the patient a second boost prolonging the effect of the surgical procedure (13). In many centres the timing of second-side operation is determined by the surgeon, however, we believe the decision regarding the timing of the second operation should be made in conjunction with the patient, centred on their symptoms of breathlessness rather than on objective improvements in FEV1. Allowing the patients themselves to decide when they would like to have further surgery ultimately prolongs the benefit of the operation improving the patient’s quality of life. This decision is based on the assessment of symptoms of dyspnoea and exercise capacity (11).
We suggest that the future direction of LVRS is the spontaneously ventilating non-intubated patient. This surgical approach aims to prevent the side-effects of intubated general anaesthesia whilst maintaining a more physiologic respiratory muscular, neurologic, and cardiopulmonary status in order to reduce the procedure-related barotrauma, to hasten recovery and optimise outcomes (14).
The lung target area for LVRS
Upper lobe only?
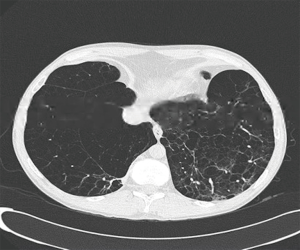
The widely held assumption from NETT is that lung volume reduction prevalent lower lobe emphysema (Figure 1) does not offer significant benefits compared to patients with predominant upper lobe prevalent emphysema undergoing LVRS (1). However, the definitions in NETT of upper and non-upper lobe predominant emphysema may not correlate with current assessment using a combination of both computed tomographic measurements (15) and perfusion scintigraphy (16). Patients with non-upper lobe predominant emphysema who undergo LVRS have a more modest benefit in comparison to patients with upper lobe predominant disease, they still however have a substantial improvement in quality of life, which may be further extended by staged bilateral LVRS (17). We have found that in patients with severe non-upper lobe predominant emphysema (Figure 2) who underwent lower lobe LVRS, FEV1 was seen to improve within 3 months from the procedure, until the second half of the first year and gradually returned to preoperative levels after 2 years. Quality-of-life improvements were mainly noted in physical components. Following our study, we conclude that performing LVRS in a carefully selected patients with non-upper lobe disease can be justified and can result in reduced morbidity with evident physiological benefits. Furthermore the concept of staged bilateral procedures can offer symptomatic relief and improvement of quality of life by ‘resetting the time’ of the natural progression of the disease (18).
No clear target areas—“homogeneous” disease
It has been observed that heterogeneous emphysema is a predictor of functional improvement. Patients with the more homogeneous type of emphysema (Figure 3) have been excluded a priori from LVRS in many centres because of the fear of removing functioning lung parenchyma, which could further compromise the patient (1).
Weder et al. concluded that well-selected symptomatic patients with severe homogeneous emphysema can also benefit from surgery. Patient who should be excluded from LVRS are those with a very low functional reserve or with pulmonary hypertension, with extreme parenchymal loss on chest CT scan, with previous recurrent infections, extensive scarring of the lungs, or previous surgery. LVRS in patients with complete homogeneous emphysema (Figure 4) can provide a comparable symptomatic and similar lung functional improvement as in patients with heterogeneous emphysema. Although the perioperative mortality for patients with homogeneous emphysema is low, their long-term survival is slightly reduced in comparison to patients with heterogeneous emphysema (19).
We believe that the mechanism by which LVRS produces functional improvement is primarily by an improvement in chest wall and diaphragm mechanics. Then in patients with severe hyperinflation and homogeneous emphysema LVRS of both upper and lower lobes can be beneficial providing there is no major loss of healthy lung parenchyma for gas exchange. The balance between improvement in chest wall mechanics and loss of functioning lung tissue must favour the former.
How best to identify target areas?
The chest CT has been routinely utilised to confirm the clinical diagnosis, the severity of emphysema and to plan the surgical procedure identifying lung areas most affected by emphysema. Complementary information to chest CT is provided by lung perfusion scintigraphy that reveals lung areas with poor perfusion and therefore poorly functioning.
The weak correlation found between chest CT and perfusion scintigraphy confirms that the two techniques work independently measuring structure and function of the lung, respectively, and therefore provide an enhanced clinical picture. In patients for LVRS, lung perfusion provides additional information to chest CT which is a purely anatomical measure of emphysema distribution whereas lung perfusion also reflects regional lung function. Therefore evaluation with CT chest and perfusion scintigraphy allow better to identify patients with a high chance of benefit from LVRS at a low risk of complications, and to obtain the information necessary to plan and perform the surgical procedure (20). Thus, allowing for a more targeted surgical approach optimising functional capacity (21).
Thurnheer et al. (22) concluded that lung perfusion scintigraphy may help to identify target areas for resection in candidates for LVRS with otherwise homogeneous emphysema. In certain patients in whom the chest CT demonstrates a homogeneous distribution of emphysematous destruction in all lobes, perfusion scintigraphy may prove to be of help by selecting target areas with relative hypo-perfusion for resection and the amount of lung volume to be resected.
Chandra et al. (16) concluded that surgical resection of emphysematous and poorly perfused lung produces better results than resection of emphysematous but better perfused parenchyma. Perfusion scintigraphy should therefore also be considered in the evaluation for LVRS in patients with upper lobe—predominant emphysema as patients with upper lung perfusion less than 20% are not only likely to live longer but also have more frequent improvement in functional outcomes with LVRS rather than continued medical management alone.
We believe that a combination of chest CT and a perfusion scintigraphy study can result in a better summation of emphysema distribution, aiding patient selection for LVRS, rather than structural disease alone.
The risk of LVRS
Although LVRS leads to improved survival in appropriately selected individuals (9), there is, we believe, an overestimation of the risks associated with the procedure, with a reported peri-operative mortality rate of 5.2% in those without high risk, and 7.9% in all patients (1). This may explain the low referral rate for assessment (23); it is estimated there are 16,000 patients potentially eligible for LVRS in the UK (24), yet only 89 LVRS procedures were performed in the UK in between 2013 and 2014 (Society for Cardiothoracic Surgery in Great Britain & Ireland—SCTS Thoracic Registry. www.scts.org/professionals/audit_outcomes/thoracic.aspx).
In order to provide a more patient-specific risk analysis we have identified pre-operative factors predictive of peri-operative mortality following LVRS. We identified the three most important independent factors to be: BMI = B, FEV1 = F and Gas Transfer = G. Each factor was assigned a risk score based on its beta coefficient (nearest whole number) and then added together to produce a total score—the “Glenfield BFG” score (with a range of 0–5). Three risk groups were classified: low risk (score 0–1), moderate risk (score 2–3) and high risk (score 4–5) groups. The Glenfield BFG score provides an objective and multi-dimensional system of scoring disease severity and demonstrates that even patients with severe markers of disease can have an acceptable postoperative prognosis and therefore gain significant benefit from LVRS. The Glenfield BFG risk score was prospectively validated on a further 71 patients and remained a significant predictor of time to death at 90-days with an AUROC of 0.84. Clinical benefits following LVRS were seen in all risk groups of the study and the score was successfully integrated into the LVR selection process. We hope that its wider application will provide a more accurate prediction of peri-operative mortality risk for LVRS and thus lead to an increase in the utilisation of LVRS. The Glenfield BFG score provides an individualised risk score for LVRS and may aid both clinician and patient in decision making around surgery (6).
How does LVRS compare with its alternative treatments?
Endobronchial valves
Recently, less invasive techniques for lung volume reduction have emerged such as the Endobronchial Lung Volume Reduction (EBLVR) with valves and coils (25). The One-way endobronchial valves (Zephyr, Pulmonx) induce atelectasis and reduce lung hyperinflation hence improving chest wall mechanics and increasing elastic recoil pressure (26). However an incomplete interlobar fissure and interlobar collateral ventilation (CV) may prevent the development of atelectasis after endobronchial one-way are deployed (27). CV is defined as “the ventilation of alveolar structures through passages or channels that bypass the normal airways” (28).
Assessment of CV is evaluated through invasive measurement methods such Chartis System® (PulmonX Inc., Redwood City, CA, USA) or non-invasive methods as CT-fissure analysis. The Chartis system is an invasive method consisting of a catheter with a balloon component at the distal tip. After inflation of the balloon, the airway is blocked and air can flow only through the catheter; the presence of collateral airflow is indicated by the persistence of expiratory airflow following occlusion of a lobe.
CT-fissure analysis is a non-invasive and indirect method for the measurement of CV; recently, sophisticate software tools have been developed such as the StratX™ Lung Analysis Platform, which helps to identify patients as likely responders or non-responders for EBV-treatment. The combination of the two methods most probably provides the highest accuracy and has proven to successfully predict a positive or a negative treatment response (29).
The Randomized Study of Endobronchial Valves for Advanced Emphysema Trial (VENT) demonstrated that unilateral lobar treatment with endobronchial valves, in patients with advanced heterogeneous emphysema, resulted in modest improvements of lung function, exercise tolerance and symptoms, with frequent haemoptysis and chronic obstructive pulmonary disease (COPD) exacerbations after valve implantation (30). The overall results were less impressive as not all subjects had CV excluded.
The recent Endobronchial Valve Therapy in Patients with Homogeneous Emphysema Trial (IMPACT) demonstrated that even in patients without clear target areas (homogeneous emphysema) in the absence of CV, assessed with the Chartis system, EBV therapy results in clinically significant benefits of improved lung function, exercise tolerance, and quality of life (31).
Nevertheless, we suggest that LVRS is a more physiological solution than EBV as it increases the overall lung elastic recoil by preserving the more elastic central and still healthy part of the diseased lobe. In LVRS the non-perfused, hyper-inflated lung is stapled and excised, whereas with an EBV the whole lobe, including the perfused segments, is excluded (32).
Ultimately, we believe that a good surgical outcome starts from the preoperative work-up and case selection at multidisciplinary meeting (MDT). It is important that surgery for emphysema, and particularly newer forms of treatments such as endobronchial valves and coils, should be offered only after discussion in an MDT and after informed discussion with the patient (33).
Endobronchial coils
Endobronchial coils are made of nitinol which is an alloy (nickel-titanium) that has shape-memory. The coils may work by increasing elastic recoil of lung parenchyma but as yet no physiological evidence has been released. They are deployed via a specialised catheter into the bronchial sub-segmental airway around 10 coils placed per lobe, in order to maximise re-tensioning of the airway. It prevents airway collapse and lung hyperinflation by mechanically compressing the parenchyma and shortening the airway; thereby increasing regional radial tension. Treatment with coils do not rely on the absence of CV to produce clinical improvements but pre-treatment analysis still requires chest CT scan to exclude patients with severe bullous disease, suspicious nodules, and active infection (34).
Coil treatment in homogeneous emphysema has been prospectively evaluated demonstrating improvements in exercise capacity but a less notable increase in FEV1 (35).
The Effect of Endobronchial Coils vs. Usual Care on Exercise Tolerance in Patients With Severe Emphysema Trial (RENEW) concluded that the use of endobronchial coil, in patients with severe homogeneous or heterogeneous lung emphysema, compared with usual care resulted in modest improvement of exercise tolerance and uncertain clinical importance, with a higher likelihood of major complications (36).
Questions remain concerning technical details of how many coils have to be inserted and in which lobe in patients with homogenous emphysema. Therefore, at present their use should be limited to clinical research.
Lung transplantation
Patients with severe COPD who remain symptomatic despite optimal medical therapy and whose predicted disease-related survival is no greater than the predicted survival after transplantation may be referred for lung transplantation (37). However, we suggest that LVRS should be considered before lung transplantation except in patients with established pulmonary hypertension (MPAP >40 mmHg) or hypercapnia (pCO2 >8 kPa). Even in cases where patients do not meet NETT inclusion criteria, FEV1 <20% predicted or DLCO <20% predicted LVRS may be considered as it may act as a bridge to subsequent transplant (1,38), The advantages of LVRS over lung transplantation include its application in the elderly and in those with chronic active infection and lung cancer which are contraindications for lung transplantation (39) whereas LVRS has been performed successfully and curatively in patients with limited-stage lung cancer (40). LVRS obviates the risks from postoperative medical or psychological or social factors that may interfere with the patient’s ability to maintain a consistent immunosuppressive regimen (41). Lung transplantation results in greater short-term mortality and morbidity than most LVRS, most often from graft failure and non-cytomegalovirus infections, and also in greater long-term mortality and morbidity (42),
Furthermore, LVRS is arguably more cost-effective partly attributable to a shorter postoperative in-hospital stay after LVRS of about 10 days compared with about 16–35 days for lung transplantation (43).
Conclusions
LVRS is a proven treatment of severe emphysema at the end of medical therapy. When offered in a staged unilateral VATS program after careful preoperative preparation by a multidisciplinary team it offers a sustainable benefit to a wide spectrum of patients. It has inherent physiological benefits over EBLVR and is more accessible and potentially less risky than transplantation. The risks of LVRS are commonly overstated and should be considered on an individual basis. The under-utilisation of LVRS should be addressed by all major thoracic surgical institutions.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.11.08). DAW serves as an unpaid Associate Editor-in-Chief of Shanghai Chest. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Group NETTR. A Randomized Trial Comparing Lung-Volume–Reduction Surgery with Medical Therapy for Severe Emphysema. N Engl J Med 2003;348:2059-73. [Crossref] [PubMed]
- Hoidal JR, Niewoehner DE. Pathogenesis of Emphysema. Chest 1983;83:679-85. [Crossref] [PubMed]
- Hamacher J, Büchi S, Georgescu CL, et al. Improved quality of life after lung volume reduction surgery. Eur Respir J 2002;19:54-60. [Crossref] [PubMed]
- Ries AL, Kaplan RM, Limberg TM, et al. Effects of Pulmonary Rehabilitation on Physiologic and Psychosocial Outcomes in Patients with Chronic Obstructive Pulmonary Disease. Ann Intern Med 1995;122:823-32. [Crossref] [PubMed]
- Moy ML, Ingenito EP, Mentzer SJ, et al. Health-Related Quality of Life Improves Following Pulmonary Rehabilitation and Lung Volume Reduction Surgery. Chest 1999;115:383-9. [Crossref] [PubMed]
- Greening NJ, Vaughan P, Oey I, et al. Individualised risk in patients undergoing lung volume reduction surgery: the Glenfield BFG score. Eur Respir J 2017;49:1601766 [Crossref] [PubMed]
- Brantigen OC. Surgical treatment of pulmonary emphysema. Am Surg 1957;23:789-804. [PubMed]
- Low DE, Trulock EP, Kaiser LR, et al. Morbidity, mortality, and early results of single versus bilateral lung transplantation for emphysema. J Thorac Cardiovasc Surg 1992;103:1119-26. [PubMed]
- Criner GJ, Cordova F, Sternberg AL, et al. The National Emphysema Treatment Trial (NETT) Part II: Lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med 2011;184:881-93. [Crossref] [PubMed]
- Brenner M, McKenna RJ, Gelb AF, et al. Rate of FEV1 Change Following Lung Volume Reduction Surgery. Chest 1998;113:652-9. [Crossref] [PubMed]
- Oey IF, Morgan MD, Spyt TJ, et al. Staged bilateral lung volume reduction surgery - the benefits of a patient-led strategy. Eur J Cardiothorac Surg 2010;37:846-52. [Crossref] [PubMed]
- Hazelrigg SR, Boley TM, Grasch A, et al. Surgical strategy for lung volume reduction surgery. Eur J Cardiothorac Surg 1999;16:S57-60. [Crossref] [PubMed]
- Oey IF, Waller DA, Bal S, et al. Lung volume reduction surgery – a comparison of the long term outcome of unilateral vs. bilateral approaches. Eur J Cardiothorac Surg 2002;22:610-4. [Crossref] [PubMed]
- Pompeo E. State of the art and perspectives in non-intubated thoracic surgery. Ann Transl Med 2014;2:106. [PubMed]
- Washko GR, Martinez FJ, Hoffman EA, et al. Physiological and Computed Tomographic Predictors of Outcome from Lung Volume Reduction Surgery. Am J Respir Crit Care Med 2010;181:494-500. [Crossref] [PubMed]
- Chandra D, Lipson DA, Hoffman EA, et al. Perfusion Scintigraphy and Patient Selection for Lung Volume Reduction Surgery. Am J Respir Crit Care Med 2010;182:937-46. [Crossref] [PubMed]
- Waller D, Oey I. Staged Lung Volume Reduction Surgery—Rationale and Experience. Thorac Surg Clin 2009;19:187-92. vii-viii. [Crossref] [PubMed]
- Perikleous P, Sharkey A, Oey I, et al. Long-term survival and symptomatic relief in lower lobe lung volume reduction surgery. Eur J Cardiothorac Surg 2017;52:982-8. [Crossref] [PubMed]
- Weder W, Tutic M, Bloch KE. Lung volume reduction surgery in nonheterogeneous emphysema. Thorac Surg Clin 2009;19:193-9. [Crossref] [PubMed]
- Washko GR, Hoffman E, Reilly JJ. Radiographic evaluation of the potential lung volume reduction surgery candidate. Proc Am Thorac Soc 2008;5:421-6. [Crossref] [PubMed]
- Cederlund K, Högberg S, Jorfeldt L, et al. Lung perfusion scintigraphy prior to lung volume reduction surgery. Acta Radiologica 2003;44:246-51. [Crossref] [PubMed]
- Thurnheer R, Engel H, Weder W, et al. Role of Lung Perfusion Scintigraphy in Relation to Chest Computed Tomography and Pulmonary Function in the Evaluation of Candidates for Lung Volume Reduction Surgery. Am J Respir Crit Care Med 1999;159:301-10. [Crossref] [PubMed]
- McNulty W, Jordan S, Hopkinson NS. Attitudes and access to lung volume reduction surgery for COPD: a survey by the British Thoracic Society. BMJ Open Respir Res 2014;1:e000023 [Crossref] [PubMed]
- Clark SJ, Zoumot Z, Bamsey O, et al. Surgical approaches for lung volume reduction in emphysema. Clin Med (Lond) 2014;14:122-7. [Crossref] [PubMed]
- Mineshita M, Slebos DJ. Bronchoscopic interventions for chronic obstructive pulmonary disease. Respirology 2014;19:1126-37. [Crossref] [PubMed]
- Klooster K, ten Hacken NH, Hartman JE, et al. Endobronchial Valves for Emphysema without Interlobar Collateral Ventilation. N Engl J Med 2015;373:2325-35. [Crossref] [PubMed]
- Shah PL, Herth FJF. Current status of bronchoscopic lung volume reduction with endobronchial valves. Thorax 2014;69:280-6. [Crossref] [PubMed]
- Koster TD, Slebos DJ. The fissure: interlobar collateral ventilation and implications for endoscopic therapy in emphysema. Int J Chron Obstruct Pulmon Dis 2016;11:765-73. [Crossref] [PubMed]
- Gompelmann D, Eberhardt R, Slebos DJ, et al. Diagnostic performance comparison of the Chartis System and high-resolution computerized tomography fissure analysis for planning endoscopic lung volume reduction. Respirology 2014;19:524-30. [Crossref] [PubMed]
- Group VSR. A Randomized Study of Endobronchial Valves for Advanced Emphysema. N Engl J Med 2010;363:1233-44. [Crossref] [PubMed]
- Valipour A, Slebos DJ, Herth F, et al. Endobronchial Valve Therapy in Patients with Homogeneous Emphysema. Results from the IMPACT Study. Am J Respir Crit Care Med 2016;194:1073-82. [Crossref] [PubMed]
- Chung SCS, Peters MJ, Chen S, et al. Effect of unilateral endobronchial valve insertion on pulmonary ventilation and perfusion: A pilot study. Respirology 2010;15:1079-83. [Crossref] [PubMed]
- Rathinam S, Oey I, Steiner M, et al. The role of the emphysema multidisciplinary team in a successful lung volume reduction surgery programmedagger. Eur J Cardiothorac Surg 2014;46:1021-6; discussion 6. [Crossref] [PubMed]
- Klose H, Achenbach HJ. Rationale for endobronchial coil treatment as the primary intervention for patients with severe emphysema. EMJ Respir 2014;2:74-80.
- Klooster K, ten Hacken NHT, Franz I, et al. Lung Volume Reduction Coil Treatment in Chronic Obstructive Pulmonary Disease Patients with Homogeneous Emphysema: A Prospective Feasibility Trial. Respiration 2014;88:116-25. [Crossref] [PubMed]
- Sciurba FC, Criner GJ, Strange C, et al. Effect of Endobronchial Coils vs Usual Care on Exercise Tolerance in Patients With Severe Emphysema: The RENEW Randomized Clinical Trial. JAMA 2016;315:2178-89. [Crossref] [PubMed]
- Patel N, DeCamp M, Criner GJ. Lung transplantation and lung volume reduction surgery versus transplantation in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008;5:447-53. [Crossref] [PubMed]
- Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745-55. [Crossref] [PubMed]
- Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014—An update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015;34:1-15. [Crossref] [PubMed]
- Pompeo E, De Dominicis E, Ambrogi V, et al. Quality of life after tailored combined surgery for stage I non–small-cell lung cancer and severe emphysema. Ann Thorac Surg 2003;76:1821-7. [Crossref] [PubMed]
- Limbos MM, Joyce DP, Chan CK, et al. Psychological functioning and quality of life in lung transplant candidates and recipients. Chest 2000;118:408-16. [Crossref] [PubMed]
- Trulock EP, Christie JD, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant 2007;26:782-95. [Crossref] [PubMed]
- Weinstein MS, Martin UJ, Crookshank AD, et al. Mortality and functional performance in severe emphysema after lung volume reduction or transplant. COPD 2007;4:15-22. [Crossref] [PubMed]
Cite this article as: Di Martino M, Gupta P, Waller DA. A perspective on lung volume reduction surgery for pulmonary emphysema. Shanghai Chest 2017;1:54.