
Multimodality treatment of oligometastatic non-small cell lung cancer: review of literature
Introduction
Non-small cell lung cancer (NSCLC) ranks among the most commonly malignancies in both men and women and is the leading cause of cancer-related death worldwide. Almost half of patients with NSCLC have distant metastases at presentation and this state is generally associated with a poor prognosis. However, since 1980s, several series have reported prolonged survival following complete resection of primary tumors and secondary lesions in selected patients who presented with only a limited number of metastases, so-called oligometastatic disease (1-3). Most of the studies established 1 to 5 as the cutoff of the number of secondary lesions and organs involved.
The concept of “oligometastasis” originates from two historical models of cancer progression: the Halsted model of contiguous step-wise progression, where a radical local control leads to a definitive cure of the disease, and the Fisher model, which postulated that clinically apparent disease was a manifestation of widespread systemic involvement, thus a less extensive surgery allows the same control of the disease. The oligometastatic state described by Hellman and Weichselbaum in 1995 (4) defines an intermediate state between localized and systemic disease. This concept is related to the notion that cancer patients with 1–5 metastatic or recurrent lesions that could be treated by local therapy achieve long-term survival or cure. In 2010, the concept of oligometastases was revised by Niibe which showed that the most important prognostic factor was the status of the primary lesion, speaking about into oligo-recurrence (5). Therefore in oligo-recurrence the oligometastases are metachronous; synchronous oligometastases suppose an active primary lesion. Patients with oligo-recurrence are considered to have a better prognosis than those with synchronous oligometastases (6).
In the last two decades, unlike the past, oligometastases seem relatively common, concerning about 30% of cases of metastatic lung cancer (7-9). Increased rate of oligometastatic disease detecting is probably due to the improvement in diagnostic imaging and the introduction of new techniques, like the positron emission tomography.
The treatment’s cornerstone of oligometastatic lung cancer is still represented by systemic therapy in addition to aggressive locally ablative treatments (radiotherapy or surgical resection).
We have reviewed the literature in order to clarify the different trends of treatment in oligometastatic NSCLC, particularly referring to single brain or adrenal gland metastasis.
Material and methods
We searched the database PubMed/Medline, using the keywords “lung cancer”, “oligometastasis”, “adrenal gland metastasis” and “brain metastasis”, for article in English that were published between 1975 to 2017. We excluded case reports or small series <10 patients. We also performed a manual search using the bibliography from these articles.
Results
Adrenal gland metastasis in oligometastatic disease: analysis of literature (Table 1)
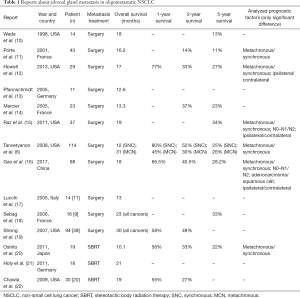
Full table
Isolated adrenal metastasis in NSCLC is estimated at 1–20% (8,23,24). Prognosis of these patients is generally poor; when metastatic disease is limited to adrenal gland, surgical resection may be proposed and seems to lengthen survival (12). By contrast, in incurable metastatic disease it seems to be useless.
Adrenalectomy represents the gold standard in the “local” treatment of isolated adrenal gland metastases if technically possible and in fitting patients (16). Nevertheless, in recent years the number of series reporting stereotactic body radiotherapy (SBRT) and radiofrequency ablation as curative treatment has increased. Several studies (20-22,25) showed the effectively and safely of these techniques, used as a noninvasive alternative to the reference standard of surgery. However, at the date, no prospective clinical trials for prognostic comparison of this treatment with surgery are available (16).
The first report, in chronological order, speaking about adrenalectomy in oligometastatic disease was carried out in 1998 by Wade (10). The mean survival for patients treated by adrenalectomy in lung cancer was 18 months, with a mean disease free interval of 14 months for metachronous lesions.
Porte et al. (11) studied 43 patients with resected NSCLC and resected solitary adrenal metastasis, in eight different centers. They observed a longer disease free survival (DFS) (17.5 vs. 5.7 months; P=0.05) and an inferior recurrence rate (68% vs. 90%; P=0.05) in patients with synchronous metastasis compared to metachronous disease with an overall survival rate of 16.2 months after metastasis resection.
Howell et al. (12) remarked a better survival rate at 1-year (64% vs. 83.3%), 2-year (21% vs. 51%) and 5-year (21% vs. 34%) and after first surgical resection (P=0.028) in patients with resected metachronous metastasis compared to patients with synchronous metastasis. The disease free interval was longer at 1-year (90% vs. 56%), 2-year (56% vs. 33%) and 5-year (39% vs. 0%) in metachronous group (P=0.038).
In 11 patients observed by Pfannschmidt et al. (13), the median overall survival (OS) after metastasectomy was 12.6 months; patients affected by metachronous disease tended to do better than patients with synchronous adrenal metastases (30.9 vs. 10.3 months).
Mercier et al. (14) evaluated their retrospective series of 23 patients. In the univariate and multivariate analysis, no survival differences were found for synchronous and metachronous disease. The only significant difference in survival rate was found about the diseases free interval <6 months for the metachronous metastasis.
All the previous papers concluded that adrenalectomy should be the treatment of choice of isolated adrenal metastasis, considering of favorable long-term survival.
Raz et al. (15) analyzed survival of 37 patients with adrenal gland metastasis from NSCLC. Surgical resection was performed in only 20 patients and the rest (17 patients) was treated non-operatively because of abdominal lymph-node involvement or extensive comorbid conditions. The 5-year OS was better in the surgical group than the non-operative group (34% vs. 0%, P=0.002) and it was influenced by two prognostic factors: ipsilateral compared with contralateral metastases relative to primary cancer (83% vs. 0%, P=0.003), N0/N1 vs. N2 primary disease (52% vs. 0%, P=0.008). The report affirmed the importance of lymph-node involvement in primary lung cancer to select the unlikely to benefit from metastatic surgery.
A systematic review of ten studies, consisting in 114 patients, was carried out by Tanvetyanon et al. (6) in 2008. The authors observed that median OS was shorter for patients with synchronous lesions than those with metachronous (12 vs. 31 months, P=0.02); the 5-year survival was equivalent into two groups (25% vs. 26%).
Gao et al. (16) carried out a pooled analysis consisting of 13 reports and 98 patients undergoing lung resection and adrenal metastasectomy; the median OS was 18 months with 1-, 2- and 5-year survival rate of 66.5%, 40.5% and 28.2%, respectively. Patients affected by metachronous disease had a better prognosis than those affected by synchronous disease.
Laparoscopic approach for adrenal metastasectomy has well been discussed in three reports. Lucchi et al. (17) observed that DFS ranged between 4 and 34 months in 11 patients treated by laparoscopic adrenalectomy and the OS after abdominal surgery ranged between 9 and 96 months. Sebag et al. (18) studied 16 patients affected by adrenal metastasis (10 synchronous and 6 metachronous) of different primary cancers (9 lung, 3 melanoma, 1 mesothelioma, kidney, colon and sarcoma). Mean OS was 23 months and the 5-year OS was 33%. Neither the site of primary tumor and metachronous/synchronous metastasis influenced survival. Strong et al. (19) evaluated the survival rate and compared laparoscopic and open adrenalectomy. The univariate analysis of all patients showed a not significant different survival at 1-year (86% vs. 73%), 3-year (46% vs. 38%) and 5-year (31% vs. 33%) between synchronous and metachronous metastasis (P=0.62). The only founded negative prognostic factor on the survival was the size >4.5 cm of adrenal metastasis (P=0.01). All three reports concluded that laparoscopic adrenal metastasectomy, when anatomically feasible, was safe in patients resected for NSCLC and it provided the additional benefits of minimally invasive surgery.
Oshiro et al. (20) spoke about feasibility of SBRT (median dose 45 Gy). The OS rates for the 19 patients at 1-, 2- and 5-year were 55.6%, 33.4% and 22.3%, respectively and the OS tended to be better for patients with metachronous metastasis (55.6% vs. 0% after 5 years).
Holy et al. (21) observed the outcomes in 18 patients who underwent SBRT (range, 40–80 Gy). The median disease free interval was 12 months (range, 2–42 months) and the OS for the isolated adrenal metastasis cohort was 23 months.
Chawla et al. (22) observed 30 patients affected by adrenal metastases from various primary site including lung (n=20), treated by SBRT (median dose 40 Gy). The OS was 19 months and the survival rate at 1- and 2-year was 42% and 25%, respectively.
Similar results were found in the report of Casamassima et al. (25), dealing with 24 patients with adrenal metastasis and lung cancer treated by SBRT (mean dose 36 Gy). In this series the overall survival was 16.2 months with 1- and 2-year OS of 39.7% and 14.5%.
The last four reports concluded that radiotherapy is safely and it contributes to improve survival but it should be considered as a treatment option in patients in whom surgery is not applicable.
Brain metastasis in oligometastatic disease: analysis of literature (Table 2)
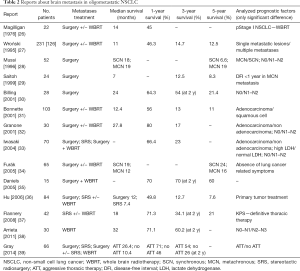
Full table
The brain is the most common site of metastasis in NSCLC and, in the absence of treatment, the median OS from diagnosis is 1 to 2 months (40). Approximately 30–50% of patients with NSCLC develop brain metastases, and they are present in about 10% of patients at initial diagnosis (41).
In patients with a solitary brain metastasis, surgical excision represents the standard treatment with low morbidity and mortality (0–3%) (28). Other options are whole brain radiotherapy (WBRT), stereotactic radiosurgery (SRS), or a combination of surgery and WBRT with improvement in local control and OS than single surgery (42).
Although the role of SRS in the recent years has been increasing, because of his minimally invasive character and similar outcomes and safety compared to surgery (43), in patients with a surgically accessible single brain metastasis, surgical excision is often considered as the gold standard treatment. WBRT or SRS should be proposed in adjuvant setting to reduce the risk of tumor recurrence or as an alternative to surgery in unfitting patients (ESMO guidelines) (44). Although no prospective randomized trials on brain oligometastatic NSCLC are present in literature, such as in adrenal metastasis treatment, the current clinical experience thus far supports an aggressive multimodal treatment.
Several retrospective studies have shown that NSCLC patients with synchronous brain metastases had improved survival times when the radical treatment to the metastases and primary tumor was performed, with a median survival of 5.2 to 64.9 months, 1-year OS of 22% to 95% and 5-year OS of 11% to 30% (26-39,45,46). When no radical treatment was administered to the primary tumor, the survival decreased.
In 1976 Magilligan (26) presented 25 years of experience with resected NSCLC and brain metastases followed by WBRT. Survival was prolonged and quality of life improved for most. The 1-year OS was 45% and freedom from significant symptoms was achieved in 55% of patients. Subsequent to this report, other centers have carried out retrospective analyses. Granone and colleagues (32) reported the 3-year OS rate of 30 cStage IV NSCLC patients with single brain metastases as 17%, and Billing and associates (30) showed a 5-year OS rate of 21.4% in 28 patients with synchronous brain metastases; in both cases the patients had been treated by local treatment for the brain metastases and pulmonary resection.
Bonnette et al. (31) reported a retrospective series of 103 patients with NSCLC with synchronous brain metastases, who underwent surgical resection of the primary lung tumor with or without perioperative chemotherapy. Treatment of metastasis was surgical resection followed by adjuvant WBRT in the 91% of patients. The 3- and 5-year OS was 13% and 11%, respectively, with a median survival of 12.4 months. Although complete resection of the primary lung cancer did not reach significance (P=0.057), it seems legitimate to proceed with lung resection after complete resection of a single brain metastasis only if complete resection is anticipated.
Gray et al. (39) analyzed data from 66 NSCLC patients with synchronous brain-only metastases. Thirty-eight patients received an aggressive thoracic therapy (surgical resection or chemoradiotherapy) which was associated with prolonged median OS (26.4 vs. 10.4 months; P<0.001) with actuarial 2-year rates of 54% vs. 26%.
One of the largest retrospective series was conducted by Hu et al. (36) and included 84 patients who received RT and/or chemotherapy (n=44, 52%) for primary lung tumor management, and surgical metastasectomy (n=53) or SRS (n=31) for metastases management. The 1-, 3- and 5-year OS was 49.8%, 12.7% and 7.6%, respectively. There was improved OS for patients receiving primary tumor treatment (median 15.5 vs. 5.9 months, P=0.046).
Similar results were reported in a more recent study (37) of 42 NSCLC patients with synchronous solitary brain metastases treated with gamma knife SRS and a Karnofsky performance status (KPS) >70%. Only 26 patients (62%) received definitive thoracic therapy. The median OS was 18 months and the 1- and 5-year OS was 71.3% and 21%, respectively. As expected, KPS and definitive thoracic therapy were significant prognostic factors on multivariate analysis.
Wroński et al. (27) in a retrospective study of 126 patients with resected NSCLC and single brain metastases, compared synchronous and metachronous disease. Eighty-four percent of patients received WBRT either before or after craniotomy (66.2% after). There was no significant difference of survival between metachronous (88 patients) and synchronous (38 patients) metastasis (19.2 vs. 12.4 months P=0.25).
Saitoh and colleagues (29) reviewed data of 24 patients with resected metachronous solitary brain metastasis. The 3- and 5-year OS rates after brain surgery were 12.5% and 8.3%, respectively. A disease-free interval of less than 1 year between surgery on the primary tumor and diagnosis of brain metastasis significantly impacted long-term survival.
Favorable survivals in brain oligometastatic NSCLCs were shown in an Australian report (35) which analysed a subgroup of 15 patients with resection of synchronous or metachronous brain metastases followed by WBRT. Complete cerebral resection was achieved in 12 cases. The 5-year OS after attempted curative resection of brain metastases and successful complete resection was 60% and 70%, respectively. In addition, studies by Iwasaki (33) and Furák (34), and their associates, did not show statistical significant difference in survival rates between metachronous brain metastases and synchronous disease.
Mussi et al. (28) investigated about OS in 52 patients with resected NSCLC and single brain metastasis. The 5-year OS was 6.6% in patients (n=19) with synchronous disease versus 19% in patients with metachronous (n=33) metastases. In the metachronous group, N0 status and interval between lung and brain operation equal to or longer than 14.5 months, significantly improved 5-year survival (61%). Billing (30), Granone (32), Iwasaki (33) and their colleagues reported that the presence of thoracic lymph node metastases (N1 or N2) significantly affected 5-year survival (P=0.001).
In a series by Arrieta et al. (38) the outcomes of 30 patients with NSCLC and synchronous brain-only metastases were analyzed. The treatment consisted of WBRT and concurrent chemoradiotherapy to primary lung tumors. Median OS and progression-free survival (PFS) were 32 and 8.5 months, respectively. The 1- and 2-year OS was 71.1% and 60.2%, respectively. Furthermore, 3-year OS was significantly superior in patients with N0-N1 disease compared to N2–N3 disease (60% vs. 24%, P=0.038).
Discussion
The cornerstone of treatment of oligometastatic disease is still represented by systemic therapy, consisting of a cisplatin or carboplatin doublet plus a third-generation agent, with a median survival in the order of 8–11 months (47), which is significantly better than after supportive care alone. However, sub-groups of patients with specific molecular and biological characteristics and a better prognosis are being identified (45,46). Local therapy (surgery and/or chemotherapy and/or radiotherapy) is mandatory to eradicate all known sites of disease (primary tumor and metastases). An aggressive local treatment is already recommended in the current version of NCCN-guidelines, where it is yet confined to brain and adrenal involvement (48). The 5-year survival of oligometastatic patients associated with this curative intent is around 32% (30,49,50), which is far greater than survival of stage IV population treated with palliative intent (5-year survival, 4%) (51). The median OS of these patients ranged between 10 and 30% in the different analyzed series.
If technically possible and in fitting patients, surgical resection represents the gold standard in the “local” treatment of isolated adrenal gland (16) and solitary brain metastasis, with low morbidity and mortality (0–3%) (28). In single-brain metastatic setting a combination of surgery and WBRT improved the local control and OS than single surgery (42).
The role of the surgery in the metastasis treatment has been largely discussed in literature and an aggressive surgical approach is more and more acknowledged in the last fifteen years, such as testified by Bartlett et al. (52) in a recent paper. From the analysis of the largest all-payer inpatient database in the United States, the authors observed the doubling of the performed brain and adrenal metastasectomies in the last decade, with more safeness despite increasing patient comorbidity.
By contrast, in the patients unfitting for surgery, other therapeutic options such as SBRT, WBRT and SRS should be considered; even if these approaches are effective and with similar outcomes and safety compared to surgery (43), they have been considered as a noninvasive alternative to the reference gold standard of surgery in not-fitting patients, because of his minimally invasive character (ESMO guidelines) (44). However, at the date, no prospective randomized trials to compare different treatments on brain or adrenal oligometastatic NSCLC are available.
The survival rate of the patients affected by isolated adrenal gland metastasis is influenced with respect to metachronous or synchronous character of metastasis: the first one is associated with longer median OS (6,10,12,15,16,20). The reasons why patients with metachronous metastases would survive longer are still unclear: although morbidity and mortality of associated thoracic interventions could be a possible explication for shorter survival in synchronous metastasis, biology of disease is probably responsible for survival differences, metachronous metastasis presenting generally in the setting of less aggressive slowly-growing cancer (6,12).
Ipsilateral or contralateral character with respect to primary lung cancer could have an impact on survival of adrenal metastasis. Thus, the question remains matter of debate, as other reports observed a better prognosis in patients with ipsilateral metastases (12,15,16). Ipsilateral character could be considered a more indolent form of lymphatic regional spread through the retroperitoneum, in contrast with more aggressive hematogenous route responsible of contralateral metastasis (53).
Histologic type had no impact on survival rate of oligometastatic patients (16). On the other hand, a trend toward worse prognosis is generally present in patients with N+ disease of primary lung tumour (15,16,54). Presence of lymph–node metastases (N1–N2) would express the aggressive character of primitive neoplasm and its tendency to give distant spread. In particular, mediastinal node involvement of primary lung cancer was confirmed to be an adverse prognostic factor for survival in patients with brain oligometastatic NSCLC in several studies (30,38,49,50,55). Based on these and similar retrospective findings, the American College of Chest Physicians currently recommends limiting curative intent treatment of oligometastatic NSCLC to patients in whom N2 disease has been excluded with invasive staging (56).
The number of metastases and the number of metastatic sites is another prominent predictor of survival in brain oligometastatic disease. In an analysis by Albain and associates (7) of 2,531 patients with advanced-stage NSCLC, the median OS was different for patients with a single lesion, patients with multiple lesions in 1 site, and patients with multiple lesions in multiple sites (8.7, 6.2 and 5.1 months, respectively). Similarly, Parikh et al. (9) in 2014 observed a better prognosis in oligometastatic disease (186 out of 725 stage IV NSCLC) compared to the population with more secondary lesions (median OS 17 vs. 14 months). Similar results were obtained in preparation for the revision of the eight edition of TNM by IASCL Lung Cancer Staging Project (8), in which tumors with a single metastasis in a single organ (M1b) showed a significantly better prognosis than those with multiple metastases in one or several organs (M1c) [median survival: 11.4 months (95% CI: 9.6–13.7) vs. 6.3 (4.8–7.0)].
All the above-mentioned prognostic factors in oligometastatic NSCLC are confirmed in a recent meta-analysis of 757 patients conducted by Ashworth et al. (55). Among other determinants, a significantly worse outcome in OS was reported for synchronous as opposed to metachronous appearance of oligometastases. This was especially the case for patients with concurrent N1/N2 disease. The authors created a model for OS, using recursive partitioning analysis, based on N-stage and metachronous/synchronous metastases (low, intermediate and high risk group). Low-risk patients (any N-stage, metachronous metastases) seemed to benefit most from aggressive oligometastatic treatment, compared to intermediate (synchronous metastases but N0 disease) and high risk (synchronous metastases and N1/N2 disease). So, patient selection plays a key role in deciding the optimal therapeutic approach.
Anyway, local treatment of metastasis in oligometastatic disease rests an argument of debate. The rationale of removal metastasis is insecure with some persisting black spots: local treatments may be of help to heal local disease, control symptoms, prevent complications and possibly, lengthen survival, but the impact on a definitive cure intention is unclear: 5-year survival rates reported in previous studies seem encouraging (16,28,31,35). Surgical resection and radiation therapy could promote immune-recovery of the disease: surgery could help to restore the immunosurveillance in the patient, while an abscopal effect of radiation therapy could be advocated (57,58).
In the last years, we have assisted to a remarkable better-than-expected OS in the oligometastatic NSCLCs, compared to classical IV stage NSCLC population [5-year survival 30% vs. 4% (30,49-51)]. The reason of this notable difference is still unclear and it rests controversies in the scientific community. Even if oligometastatic state represents a clinical-radiological condition, with a biological substrate not completed elucidated, it seems to be less aggressive and slowly-growing cancer, characterized by a totally different biological and immunological environment. In view of these last considerations and the more indolent cancer’s evolution in this subgroup of patients, an aggressive local approach seems to be not only efficacy, but even mandatory. Therefore, it is not clear if long-term survival in oligometastatic NSCLC is due to the effectiveness of the locally ablative treatment itself, or rather due to the selection of fit patients with a slow-growing malignancy.
In summary, the brain-only oligometastatic patients with NSCLC who seem to benefit most from both aggressive local and intracranial management are highly selected patients with younger age, good performance status, a good control of the primary lung tumor radically treated, a single metastatic site and without thoracic nodal involvement. In patients with synchronous brain disease, surgical metastasectomy should be performed before pulmonary resection. The alternative approach with radiosurgery is recommended for multiple brain metastases, surgically inaccessible lesions or generally medical inoperable patients. Given the more favorable prognosis and longer disease-free intervals in the metachronous oligometastatic setting, an aggressive treatment with either surgery or radiation should be administered. If/when the timing and number of metastases exceeds what can reasonably be controlled with local therapy, systemic therapy is usually warranted. In patients with adrenal metastasis of NSCLC, surgery should be considered the first treatment if primary tumour is controlled, in the absence of disseminated metastatic disease and in fitting patients. It has to be integrated in the setting of a multidisciplinary approach including surgery, chemotherapy, radiotherapy, and targeted therapies.
Conclusions
Oligometastatic disease represents a clinical and radiological condition with a biological substrate not completely elucidated, characterized by less aggressively and slow-growth, probably due to a different biological and immunological environment than stage IV lung cancer. Local aggressive treatment of primary cancer and metastasis, in association with systemic therapy, seems to be useful in preventing complications, controlling local symptoms and lengthening survival. In perspective, we think that restoring immunosurveillance by the association of local treatments and immune check points inhibitors together with supportive cares could represent a novel treatment approach of oligo-metastatic disease which needs to be evaluated in specifically designed clinical trials.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Twomey P, Montgomery C, Clark O. Successful treatment of adrenal metastases from large-cell carcinoma of the lung. JAMA 1982;248:581-3. [Crossref] [PubMed]
- Khan AJ, Metha PS, Zusag TW, et al. Long term disease-free survival resulting from combined modality management of patients presenting with oligo-metastatic, non-small cell lung carcinoma (NSCLC). Radiother Oncol 2006;81:163-7. [Crossref] [PubMed]
- Pfannschmidt J, Dienemann H. Surgical treatment of oligo-metastatic non-small cell lung cancer. Lung Cancer 2010;69:251-8. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol 2010;40:107-111. [Crossref] [PubMed]
- Tanvetyanon T, Robinson LA, Schell MJ, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: a systematic review and pooled analysis. J Clin Oncol 2008;26:1142-7. [Crossref] [PubMed]
- Albain KS, Crowley JJ, LeBlanc M, et al. Survival determinants in extensive-stage non-small cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol 1991;9:1618-26. [Crossref] [PubMed]
- Eberhardt WEE, Mitchell A, Crowle J, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the M descriptors in the forthcoming eight edition of the TNM classification of lung cancer. J Thorac Oncol 2015;10:1515-22. [Crossref] [PubMed]
- Parikh RB, Cronin AM, Kozono DE, et al. Definitive primary therapy in patients presenting with oligometastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2014;89:880-7. [Crossref] [PubMed]
- Wade TP, Longo WE, Virgo KS, et al. A comparison of adrenalectomy with other resections for metastatic cancers. Am J Surg 1998;175:183-6. [Crossref] [PubMed]
- Porte H, Siat J, Guibert B, et al. Resection of adrenal metastases from non-small cell lung cancer: a multicenter study. Ann Thorac Surg 2001;71:981-5. [Crossref] [PubMed]
- Howell GM, Carty SE, Armstrong MJ, et al. Outcome and prognostic factors after adrenalectomy for patients with distant adrenal metastasis. Ann Surg Oncol 2013;20:3491-6. [Crossref] [PubMed]
- Pfannschmidt J, Schlolaut B, Muley T, et al. Adrenalectromy for solitary adrenal metastases from non-small cell lung cancer. Lung Cancer 2005;49:203-7. [Crossref] [PubMed]
- Mercier O, Fadel E, de Perrot M, et al. Surgical treatment of solitary adrenal metastasis from non-small cell lung cancer. J Thorac Cardiovasc Surg 2005;130:136-40. [Crossref] [PubMed]
- Raz DJ, Lanuti M, Gaissert HC, et al. Outcomes of patients with isolated adrenal metastasis from non-small cell lung carcinoma. Ann Thorac Surg 2011;92:1788-92; discussion 1793.
- Gao XL, Zhang KW, Tang MB, et al. Pooled analysis for surgical treatment for isolated adrenal metastasis and non-small cell lung cancer. Interact Cardiovasc Thorac Surg 2017;24:1-7. [Crossref] [PubMed]
- Lucchi M, Dini P, Ambrogi MC, et al. Metachronous adrenal masses in resected non-small cell lung cancer patients: therapeutic implications of laparoscopic adrenalectomy. Eur J Cardiothorac Surg 2005;27:753-6. [Crossref] [PubMed]
- Sebag F, Calzolari F, Harding J, et al. Isolated adrenal metastasis: the role of laparoscopic surgery. World J Surg 2006;30:888-92. [Crossref] [PubMed]
- Strong VE, D’Angelica M, Tang L, et al. Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol 2007;14:3392-400. [Crossref] [PubMed]
- Oshiro Y, Takeda Y, Hirano S, et al. Role of radiotherapy for local control of asymptomatic adrenal metastasis from lung cancer. Am J Clin Oncol 2011;34:249-53. [Crossref] [PubMed]
- Holy R, Piroth M, Pinkawa M, et al. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol 2011;187:245-51. [Crossref] [PubMed]
- Chawla S, Chen Y, Katz AW, et al. Stereotactic body radiotherapy for treatment of adrenal metastases. Int J Radiat Oncol Biol Phys 2009;75:71-5. [Crossref] [PubMed]
- Higashiyama M, Doi O, Kodama K, et al. Surgical treatment of adrenal metastasis following pulmonary resection for lung cancer: comparison of adrenalectomy with palliative therapy. Int Surg 1994;79:124-9. [PubMed]
- Porte HL, Roumilhac D, Graziana JP, et al. Adrenalectomy for a solitary adrenal metastasis from lung cancer. Ann Thorac Surg 1998;65:331-5. [Crossref] [PubMed]
- Casamassima F, Livi L, Masciullo S, et al. Stereotactic radiotherapy for adrenal gland metastases: University of Florence experience. Int J Radiat Oncol Biol Phys 2012;82:919-23. [Crossref] [PubMed]
- Magilligan DJ Jr, Rogers JS, Knighton RS, et al. Pulmonary neoplasm with solitary cerebral metastasis. Result of combined excision. J Thorac Cardiovasc Surg 1976;72:690-8. [PubMed]
- Wroński M, Arbit E, Burt M, et al. Survival after surgical treatment of brain metastases from lung cancer: a follow-up study of 231 patients treated between 1976 and 1991. J Neurosurg 1995;83:605-16. [Crossref] [PubMed]
- Mussi A, Pistolesi M, Lucchi M, et al. Resection of single brain metastasis in non-small-cell lung cancer: prognostic factors. J Thorac Cardiovasc Surg 1996;112:146-53. [Crossref] [PubMed]
- Saitoh Y, Fujisawa T, Shiba M, et al. Prognostic factors in surgical treatment of solitary brain metastasis after resection of non-small-cell lung cancer. Lung Cancer 1999;24:99-106. [Crossref] [PubMed]
- Billing PS, Miller DL, Allen MS, et al. Surgical treatment of primary lung cancer with synchronous brain metastases. J Thorac Cardiovasc Surg 2001;122:548-53. [Crossref] [PubMed]
- Bonnette P, Puyo P, Gabriel C, et al. Surgical management of non-small cell lung cancer with synchronous brain metastases. Chest 2001;119:1469-75. [Crossref] [PubMed]
- Granone P, Margaritora S, D’Andrilli A, et al. Non-small cell lung cancer with single brain metastasis: the role of surgical treatment. Eur J Cardiothorac Surg 2001;20:361-6. [Crossref] [PubMed]
- Iwasaki A, Shirakusa T, Yoshinaga Y, et al. Evaluation of the treatment of non-small cell lung cancer with brain metastasis and the role of risk score as a survival predictor. Eur J Cardiothorac Surg 2004;26:488-93. [Crossref] [PubMed]
- Furák J, Troján I, Szöke T, et al. Lung cancer and its operable brain metastasis: survival rate and staging problems. Ann Thorac Surg 2005;79:241-7. [Crossref] [PubMed]
- Daniels M, Wright GM. Complete resection of non-small-cell lung cancer and oligo-metastatic brain disease. ANZ J Surg 2005;75:963-66. [Crossref] [PubMed]
- Hu C, Chang EL, Hassenbusch SJ 3rd, et al. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer 2006;106:1998-2004. [Crossref] [PubMed]
- Flannery TW, Suntharalingam M, Regine WF, et al. Long-term survival in patients with synchronous, solitary brain metastasis from non-small-cell lung cancer treated with radiosurgery. Int J Radiat Oncol Biol Phys 2008;72:19-23. [Crossref] [PubMed]
- Arrieta O, Villareal-Garza C, Zamora J, et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastases treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol 2011;6:166. [Crossref] [PubMed]
- Gray PJ, Mak RH, Yeap BY, et al. Aggressive therapy for patients with non-small cell lung carcinoma and synchronous brain-only oligometastatic disease is associated with long-term survival. Lung Cancer 2014;85:239-44. [Crossref] [PubMed]
- Zimm S, Warnpler GL, Stablein D. Intracerebral metastases in solid tumor patients. Natural history and results of treatment. Cancer 1981;48:384-94. [Crossref] [PubMed]
- Schuette W. Treatment of brain metastases from lung cancer: chemotherapy. Lung Cancer 2004;45:S253-S257. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Alexander E, Moriarty TM, Davis RB, et al. Stereotactic radiosurgery for the definitive, non-invasive treatment of brain metastases. J Natl Cancer Inst 1995;87:34-40. [Crossref] [PubMed]
- Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:iii27-39. [Crossref] [PubMed]
- Mok TS. Personalized medicine in lung cancer: what we need to know. Nat Rev Clin Oncol. 2011;8:661-8. [Crossref] [PubMed]
- Alifano M, Mansuet-Lupo A, Lococo F, et al. Systemic Inflammation, Nutritional Status and Tumor Immune Microenvironment Determine Outcome of Resected NSCLC. PLoS One 2014;9:e106914. [Crossref] [PubMed]
- Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii56-64. [Crossref] [PubMed]
- Available online: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Mordant P, Arame A, De Dominicis F, et al. Which metastasis management allows long-term survival of synchronous solitary M1b non-small cell lung cancer? Eur J Cardiothorac Surg 2012;41:617-22. [Crossref] [PubMed]
- Congedo MT, Cesario A, Lococo F, et al. Surgery for oligometastatic non-small cell lung cancer: long-term results from a single center experience. J Thorac Cardiovasc Surg 2012;144:444-52. [Crossref] [PubMed]
- Ozkaya S, Findik S, Dirican A, et al. Long-term survival rates of patients with stage IIIB and IV non-small cell lung cancer treated with cisplatin plus vinorelbine or gemcitabine. Exp Ther Med 2012;4:1035-8. [Crossref] [PubMed]
- Bartlett EK, Simmons KD, Wachtel H, et al. The rise in metastasectomy across cancer types over the past decade. Cancer 2015;121:747-57. [Crossref] [PubMed]
- Károlyi P. Do adrenal metastases from lung cancer develop by lymphogenous or hematogenous route? J Surg Oncol 1990;43:154-6. [Crossref] [PubMed]
- Collaud S, Stahel R, Inci I, et al. Survival of patients treated surgically for synchronous single-organ metastatic NSCLC and advanced pathologic TN stage. Lung Cancer 2012;78:234-8. [Crossref] [PubMed]
- Ashworth AB, Senan S, Palma DA, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer 2014;15:346-55. [Crossref] [PubMed]
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-e399S.
- Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol 2009;10:718-26. [Crossref] [PubMed]
- Bobbio A, Alifano M. Immune therapy of non-small cell lung cancer. The future. Pharmacol Res 2015;99:217-22. [Crossref] [PubMed]
Cite this article as: Mazzella A, Mastromarino MG, Zhang X, Bobbio A, Alifano M. Multimodality treatment of oligometastatic non-small cell lung cancer: review of literature. Shanghai Chest 2017;1:63.


