
Video-assisted thoracic surgery (VATS) major pulmonary resections: different approaches and focus on the full thoracoscopic fissure-based technique
The development and adoption of major pulmonary resections (MPR) by video-assisted thoracic surgery (VATS) have long been limited by technical difficulties, vascular risks and the reluctance of a part of the surgical community (1). In some way, resistance was understandable since, at the very onset of this new surgery, image quality was poor and dedicated instrumentation was lacking. Surgeons involved in this surgery took several years to develop a reliable, safe and reproducible technique. This preamble aims at explaining why high-volume expert centers only trust the technique they have worked hard to develop and don’t consider moving towards other modes of operation. The authors of these lines is no exception. This article will therefore be influenced by the technique developed and used for many years in our institution by all the surgeons of our team.
In 2017, the various techniques and approaches used in video-assisted MPR can be summarized as follows:
- Hybrid approach combines a muscle-sparing thoracotomy whose length varies from 4 to 10 cm, with both direct and thoracoscopic vision (2). Some of the promotors of this technique argue it is well suited to segmentectomies because it achieves adequate tumor-free margins and helps in intersegmental plane determination;
- In the multiport approach, several trocars (up to 4 or 5) are used, one for the endoscope and the others for instruments. Some surgeons add a so-called utility incision (3 to 6 cm) (3) while other perform a full thoracoscopic approach, also known as complete-VATS (4). However, when looking at the line drawings and photographs illustrating the corresponding articles reporting these techniques, there is a wide range of sizes and locations of ports;
- In the single-port approach, the endoscope and instruments are inserted via a single incision, aiming at reducing port-related intercostal injury and pain (5);
- Between single and multiple ports, many techniques using 2 or 3 trocars have been reported (6). Multiple ports techniques differ by the number, size and location of ports and by the use or non-use of an access incision. Many variants of this approach are being published, including the use of micro-instruments and subxiphoid incision which can be multi-portal (7) or mono-portal (8), a recent attempt to reduce chest trauma to the minimum;
- The robotically assisted technique, is a multiple-port approach that adds degrees of freedom to instruments. It uses 3 to 4 trocars depending on the robot generation (3 or 4 arms) (9). It is interesting to note that the robotic technique, which is considered the most innovative, the most sophisticated (and the most expensive), is finally the one that comes closest to conventional surgery by its classical way of dissecting anatomical structures;
- In addition to these several approaches, the technique varies significantly, depending on a “fissure-first” or a “fissure-last” dissection;
- The fissure-first technique could be named “conventional”, with a dissection of the bronchovascular elements in the fissure, contrary to the fissure-last one where the elements are controlled in the hilum with stapling of the fissure at the end (3). The fissure-based technique has been considered as challenging, especially in case of fused fissure. However- most likely with increasing experience and improvement of technology—it has been recently demonstrated that complete fissures are not an obstacle for the use of this approach (10);
- In the fissure-last technique, once the lobar bronchus and vein have been controlled, the fissure and arterial branches are stapled “en-masse”, more or less as initially reported by Ralph Lewis in the 1990s under the name “VATS simultaneously stapled lobectomy” (11). The procedure is relatively rapid and has several advantages: oozing and air-leaks related to the opening of the fissure are minimized;
- Recently, several studies have tried to compare the single-port technique to others. Most published results raise questions. A recent prospective randomized study by Perna et al. has compared single port to multiple ports techniques (12). The authors, basing on 50 patients in each group, found no difference in postoperative pain, morphine use, chest drainage duration, complications and hospital stay. Shen et al. conducted a similar study which concluded to the superiority of uniportal approach, even though bleeding, duration of chest drainage, outcome and morbidity were similar in both groups (13). The only reported difference in favor of single port was a shorter operation time. Decaluwé stressed the limitations of such studies, not only because these are underpowered but also because of some statistical insufficiency, such as the use of inappropriate statistical tests for measurement of pain relief (14). Other authors have underlined the weakness of these comparisons (15-17). Hereafter, we list the numerous variables that make a comparison “Uni-portal” versus “Multi-portal” highly questionable (Table 1):
Table 1
Summary of all missing technical details for various techniques of thoracoscopic major pulmonary resections, explaining the limitations of comparative studiesTechnique Unreported technical details Single-port Length and location of incision Use or non-use of an additional port for scope insertion Multiple ports (full thoracoscopic) Exact number of ports Exact location of ports Diameter of ports and instruments Multiple ports with utility incision Exact number of ports Exact location of ports Diameter of ports Length and location of utility incision Robotically-assisted Use of an additional port (for stapler, retraction device etc.) Utility incision Pressure and torqueing exerted on incisions by the robotic arms For all techniques Type of scope (diameter, vision angle) Type and diameter of instruments Surgical experience Type of intra and postoperative analgesia - In a uniportal approach, what are the length of incision, location of incision, type of instruments? Is a rib spreader used? If a second trocar is used for the endoscope insertion, as often observed in some techniques (17), is it still a single-port? or should it rather be called two-ports?
- In a multiportal approach, what is the exact number of ports (3 to 5), the diameter of ports and instruments (the larger, the more painful), their location (the more posterior, the more painful)? Is there an access incision? If so, which size? which location? As underlined by Hansen et al., we should consider the intercostal space is around 1 cm and we could decrease trauma by using 5-mm instruments (16), which is what we do and what others do suggest (18). In our department, some instruments are only 3 mm in diameter. Should these be counted in the total number of ports? As written by Hansen et al., we are not yet able to demonstrate that’s “Several instruments via one incision are less painful than two or several small incisions with one instrument in each?” (16);
- In whatever approach what is the experience of the surgeon, a major factor as recently stressed (19), the duration of the procedure, the conversion rate, the type of scope (0°, 30°, 45°, deflectable) because the later factor impacts on the need of exerting pressure or torqueing on trocar. How is the chest drainage managed (number, location, diameter, use or not of suction, duration)? Is there a preoperative, intraoperative analgesia and which kind of type of postoperative analgesia is being used? In addition, randomized controlled trials (RCTs) don’t consider factors that can’t be figured out: comfort of the surgeon, stiffness issues, exposure and image quality, safety, aptitude to control intraoperative problems and complications…
Eventually, as recently pointed out, it seems we forget we are treating patients presenting with a very serious disease, i.e., lung cancer (16). This means the main concern of patients is to be cured rather than experiencing more or less pain, staying 1 or 2 days more in hospital and checking the number and length of incisions. In this matter, it is somewhat striking watching at so many presentations showing a picture of the incision side by side with a measuring tape aiming at demonstrating that access is minimal, as if this should be the ultimate goal of the procedure.
Special focus on the full thoracoscopic fissure-based technique
The technique we have reported in several publications (4,20-22) is the one used routinely in our department by all surgeons and it is almost unchanged since 2007. It is the fruit of a maturation process after using many different techniques before 2007, none of which we were satisfied with. The only notable changes in recent years concern the development of dedicated instrumentation and the use of a robotic scope holder (22,23). The technique has many advantages and some limitations that are summarized in Table 2.
Table 2
| Advantages |
| Exposure quality |
| Rapid and easy overcoming of intraoperative problems |
| Global vision |
| Natural and familiar vision |
| Reduction of blind areas by use of a deflectable scope |
| Ergonomic positioning of instruments |
| Used of dedicated instrumentation whose size is suited to the requirements of endoscopic dissection |
| No large incision (utility incision) |
| Stable image by use of a scope holder |
| Limitations |
| 3–4 ports might be more invasive than 1–2 ports |
| Longer operative time in case of fused fissure |
| Impossibility to use conventional instruments (no access incision) |
| Cost of deflectable scope |
| Cost of dedicated instruments kit |
Rational of a fissure-based dissection
The technique is very like that described by Richards et al., i.e., a posterior approach based on dissection of the broncho-vascular elements in the fissure (3). It can be summarized as a conventional technique where the surgeon stands in the patient’s back and has a familiar vision, comparable to open thoracotomy. Extensive dissection in the fissure minimizes anatomical errors, as shown in Figure 1. The popular so-called anterior approach with fissure-last technique offers several advantages (24). When the fissure is partly of totally fused (Craig-Walker Grade III or IV) (25), it avoids a tedious opening of the fissure and its potential side effects. This results in a procedure that is usually faster than the fissure-first one and safe because stapling minimizes blood loss and air leaks (26).
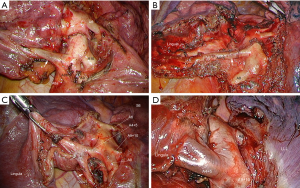
Rational of using multiple ports
The anatomical variations of the bronchi, pulmonary arteries and veins are numerous, especially in the fissures. The argument that a primary dissection of the fissure is tedious and a cause of hemorrhagic oozing and aerial leakage is not fully justified if a suitable instrumentation is used. In the case of complete fissure, two techniques can be proposed: a thorough opening of the fissure with a bipolar sealing device (27) or the tunnel technique proposed by Decaluwe et al. (28). Besides, with the current development of thoracoscopic anatomical sublobar resections, it becomes difficult to achieve a safe operation without an extensive dissection of vascular elements in the fissure.
Finally, we can observe that the promoters of a robotically assisted technique put forward the argument of multiple ports as a gain in quality and safety. It is unclear why thoracoscopic surgery would follow an inverse direction with a minimal number of ports. This observation is true today but could obviously be called into question if future technological developments made it possible to operate through a single incision with a flexible optic and fine steerable instruments. However, to date, we have not yet reached that point. Whenever possible, the diameter of trocars is reduced to the minimal, i.e., 5 and 3 mm. The reason of working with small instruments is twofold: (I) minimizing intercostal trauma and (II) enhancing the precision of dissection because instruments tips are better suited to the dimensions of the anatomical elements that are dissected.
Robot-assisted surgery is also a multiport surgery, although more sophisticated and expensive than standard thoracoscopic surgery. Compared to the latter, it has above all an ergonomic advantage and, theoretically, a higher precision due to the degrees of freedom of instruments. The comparison with conventional thoracoscopic surgery is very difficult to make and the results are contradictory. Recently a comparative study—robot versus thoracoscopy—was stopped due to more complications in the robot group (29). It is difficult to say whether these complications are due to the technique itself or to the fact that surgeons experienced in conventional thoracoscopy have had difficulty switching to the robot. Other multicenter studies involving a large number of patients have reported no significant difference between robotics and thoracoscopy (30) or conversely, an advantage for the robot in terms of complications, conversion rates and hospital stay (31). However, in this last study, it is surprising to note that the conversion rate of the thoracoscopy group was 13.1% (compared to 6.3% in the robot group). This conversion rate is much higher than that observed in large European series (5% on average) and raises the question of surgeons’ expertise in this study. Eventually, comparison should probably be conducted between high volume VATS and high-volume robot-assisted thoracic surgery (RATS) centers since—up to now—none of these centers are experts in both approaches.
Rational of not using a utility incision
Since we only use fine instruments, introduced by several trocars, we do not need to use an access incision. Even if an incision must be made at the end of the procedure to retrieve the removed lobe or segment, the length can be adapted to the size of the specimen and can sometimes be very small. Finally, we do not consider this access incision to be a security guarantee because it is rarely located in relation to the exact position of the complication to be managed. In the event of a major complication, it is preferable to convert the procedure into a true thoracotomy. As shown by Yamashita et al., when serious bleeding occurs during a VATS approach, the surgeon ends up with both an enlarged mini-thoracotomy plus a conversion into a regular thoracotomy (32).
Technological adjuncts
For years, we use a deflectable scope mounted on a scope holder. The scope houses a chip at its tip rending a High-definition image. The distal part can be deflected from 0° to 100° up-down and right-left or any combination of these movements. It is especially helpful during lymph node dissection (23).
The scope holder allows avoiding a shaking picture and keeps the operative field remains hand-free, thus preventing instruments conflicts and hands crowding over the patient’s chest. The combination of the movements of this scope holder and of the view angles of the deflectable thoracoscope makes it possible to reach most targets with minimal manipulation of the scope.
Additional minor or sophisticated tips help keeping a clear vision all along the procedure (23): (I) built-in-scope warming system that prevents fogging (Olympus) and (II) a specific trocar which deflects the blood drops, thus avoiding soiling the scope distal lens.
In addition, a wide range of dedicated instruments have been developed in our hospital to match the following requirement: one task-one tool.
In conclusion, the development of thoracoscopic techniques applied to MPR is accelerating sharply after a long period of uncertainty and reluctance. The techniques are multiple and varied, depending on the number, size and position of the trocars, optics and instruments used. Publications on this subject have numerous biases that do not allow a rational comparison. We should not ignore the fact we are operating on patients with bronchial cancer, i.e., a potentially serious disease. The crucial point is working safely and obtaining optimal oncological results. In this respect, the technique reported in this article appears to be a response—at least partial—to these concerns.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lorenzo Spaggiari and Domenico Galetta) for the series “Minimally Invasive Thoracic Oncological Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.03.06). The series “Minimally Invasive Thoracic Oncological Surgery” was commissioned by the editorial office without any funding or sponsorship. D Gossot is consultant for Delacroix- Chevalier instruments manufacturer. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yim AP. Video-assisted thoracic lung surgery: is there a barrier to widespread adoption? Ann Thorac Surg 2010;89:S2112-3. [Crossref] [PubMed]
- Yamamoto H, Shirahashi K, Matsumoto M, Miyamaoto Y, Doi K, Iwata H. Hybrid approach for VATS pulmonary resection. Video-assist Thorac Surg 2017;2:40. [Crossref]
- Richards JM, Dunning J, Oparka J, et al. Video-assisted thoracoscopic lobectomy: the Edinburgh posterior approach. Ann Cardiothorac Surg 2012;1:61-9. [PubMed]
- Gossot D. Atlas of endoscopic major pulmonary resections. 2nd edition. Springer-Verlag, 2018.
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Wang GS, Wang Z, Wang J, et al. Biportal complete video-assisted thoracoscopic lobectomy and systematic lymphadenectomy. J Thorac Dis 2013;5:875-81. [PubMed]
- ElSaegh MM, Ismail NA, Mydin MI, et al. Subxiphoid uniportal lobectomy. J Vis Surg 2017;3:24. [Crossref] [PubMed]
- Suda T. Subxiphoid Uniportal Video-Assisted Thoracoscopic Surgery Procedure. Thorac Surg Clin 2017;27:381-6. [Crossref] [PubMed]
- Park BJ. Robotic lobectomy for non-small cell lung cancer: long-term oncologic results. Thorac Surg Clin 2014;24:157-62. [Crossref] [PubMed]
- Samejima J, Mun M, Matsuura Y, et al. Thoracoscopic anterior 'fissure first' technique for left lung cancer with an incomplete fissure. J Thorac Dis 2016;8:3105-11. [Crossref] [PubMed]
- Lewis RJ, Caccavale RJ, Sisler GE, et al. VATS simultaneously stapled lobectomy. Ann Thorac Surg 1997;64:1869-71. [PubMed]
- Perna V, Carvajal A, Torrecilla J, et al. Uniportal video-assisted thoracoscopic lobectomy versus other video-assisted thoracoscopic lobectomy techniques: a randomized study. Eur J Cardiothorac Surg 2016;50:411-5. [Crossref] [PubMed]
- Shen Y, Wang H, Feng M, et al. Single versus multiple-port thoracoscopic lobectomy for lung cancer: a propensity-matched study. Eur J Cardiothorac Surg 2016;49:i48-53. [PubMed]
- Decaluwé H. One, two, three or four ports… does it matter? Priorities in lung cancer surgery. J Thorac Dis 2016;8:E1704-8. [Crossref] [PubMed]
- McElnay PJ, Molyneux M, Krishnadas R, et al. Pain and recovery are comparable after either uniportal or multiport video-assisted thoracoscopic lobectomy: an observation study. Eur J Cardiothorac Surg 2015;47:912-5. [Crossref] [PubMed]
- Hansen HJ, Varela G, Petersen RH, et al. Does the number of incisions in video-assisted thoracoscopic surgery matter? J Thorac Dis 2016;8:E1625-7. [Crossref] [PubMed]
- Perna V, Carvajal AF, Torrecilla JA, et al. Scientific rigour must come first. Eur J Cardiothorac Surg 2017;51:397. [PubMed]
- Ismail NA, Elsaegh M, Dunning J. Novel Techniques in Video-assisted Thoracic Surgery (VATS) Lobectomy. Surg Technol Int 2015;26:206-9. [PubMed]
- Wood DE. What is most important in improving outcomes after pulmonary lobectomy: the surgeon or the approach? Eur J Cardiothorac Surg 2013;43:817-9. [Crossref] [PubMed]
- Gossot D, Lutz JA, Grigoroiu M, et al. Unplanned Procedures During Thoracoscopic Segmentectomies. Ann Thorac Surg 2017;104:1710-7. [Crossref] [PubMed]
- Gossot D, Zaimi R, Fournel L, et al. Totally thoracoscopic pulmonary anatomic segmentectomies: technical considerations. J Thorac Dis 2013;5:S200-6. [PubMed]
- Seguin-Givelet A, Traibi A, Grigoroiu M, et al. Full thoracoscopic fissure based technique for major pumonary resections: rational and basic considerations. Video-assist Thorac Surg 2017;2:39. [Crossref]
- Gossot D, Grigoroiu M, Brian E, et al. Technical means to improve image quality during thoracoscopic procedures. J Vis Surg 2017;3:53. [Crossref] [PubMed]
- Hansen HJ, Petersen RH, Christensen M. Video-assisted thoracoscopic surgery (VATS) lobectomy using a standardized anterior approach. Surg Endosc 2011;25:1263-9. [Crossref] [PubMed]
- Craig SR, Walker WS. A proposed anatomical classification of the pulmonary fissures. J R Coll Surg Edinb 1997;42:233-4. [PubMed]
- Petersen RH, Hansen HJ. Learning thoracoscopic lobectomy. Eur J Cardiothorac Surg 2010;37:516-20. [Crossref] [PubMed]
- Longo F, Crucitti P, Quintarelli F, et al. Bipolar sealing devices in video-assisted thoracic surgery. J Vis Surg 2017;3:13. [Crossref] [PubMed]
- Decaluwe H, Sokolow Y, Deryck F, et al. Thoracoscopic tunnel technique for anatomical lung resections: a 'fissure first, hilum last' approach with staplers in the fissureless patient. Interact Cardiovasc Thorac Surg 2015;21:2-7. [Crossref] [PubMed]
- Park SY, Suh JW, Narm KS, et al. Feasibility of four-arm robotic lobectomy as solo surgery in patients with clinical stage I lung cancer. J Thorac Dis 2017;9:1607-14. [Crossref] [PubMed]
- Swanson SJ, Miller DL, McKenna RJ Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Yamashita S, Tokuishi K, Moroga T, et al. Totally Thoracoscopic Surgery and Troubleshooting for Bleeding in Non-Small Cell Lung Cancer. Ann Thorac Surg 2013;95:994-9. [Crossref] [PubMed]
Cite this article as: Gossot D, Seguin-Givelet A. Video-assisted thoracic surgery (VATS) major pulmonary resections: different approaches and focus on the full thoracoscopic fissure-based technique. Shanghai Chest 2018;2:15.


