
Robotic assisted lobectomy and lymphadenectomy “different approaches”
Introduction
Lobectomy associated to a radical lymphadenectomy is considered the gold standard therapy for patients with early stage non-small cell lung cancer (NSCLC) (1). With the ever-expanding screening programs as well as the greater attention of patients to their health, the number of lung cancer in the early stage is gradually increased, and associated to the development of new surgical techniques has progressively led pulmonary surgery to become less and less invasive. Despite minimally invasive approach in thoracic surgery has already proven advantages in terms of reduced postoperative pain, shorter immune response, quicker resumption of daily activities, and better aesthetic and functional result (2-7), video-assisted thoracic surgery (VATS) lobectomy is slowly becoming the standard approach to early-stage lung cancer treatment; this was probably related to technical limitations, such as two-dimensional vision, lack of instrument flexibility with difficult hand-eye coordination and long-lasting learning curve, in particular performing radical mediastinal lymphadenectomy, which is the standard of care in the treatment of lung cancer (8-13) and highly related to the long-term outcome. To address the limitations of conventional VATS, a telesurgical system was developed offering surgeons the benefits of three-dimensional high-definition imaging, greater hand movements using wristed instruments, and a computer-assisted scaling down of motion with reduction of hand-related tremors (da Vinci system, Intuitive Surgical, Sunnyvale, CA, USA), offering surgeons an innovative approach to lung cancer resection and staging, with a more precise dissection and theoretically better oncological results.
Robot-assisted thoracic surgery (RATS) is a relatively new technique for minimally invasive lung lobectomy, introduced in the operating room for pulmonary resection only in 2002. The first preliminary reports on pulmonary resection performed by RATS were published by Melfi et al. in 2002 and Giulianotti et al. in 2003, showing the clinical feasibility of the technique with encouraging results (14,15). The feasibility and safety of the robotic technique were corroborated then by other early publications including those by Park et al. (16) on 34 lung cancer lobectomies published in 2006 and by Giulianotti et al. (17) on 38 lung resections published in 2010. Afterwards, Melfi et al. (18) was the first to describe a series of 107 robotic lobectomies with lymphadenectomy performed in ‘good risk’ oncologic patients, with good results in terms of complications, number of conversions and duration of surgery.
Since then, robotic resection is gaining popularity and acceptance among different minimally invasive techniques in the thoracic community. Indeed, according to the Nationwide Inpatient Sample (NIS) database (19) published by Paul et al. in 2014, the number of centers performing RATS lobectomy and the rate of RATS compare to all lobectomies per center increased dramatically in the last 10 years. The percentage of robotic lobectomies performed per year in US went from 1% in 2008 to 48.8% in 2011, compare to a stable proportion of VATS lobectomy which has been maintained around 20–30% over the years.
Robotic technology
So far, the Da Vinci Surgical System is the only robotic system approved and available on market for RATS. Da Vinci Surgical System is composed by a surgeon console, patient-side cart, EndoWrist instruments, and vision system. The surgeon operates seated comfortably at a console, some distance from the patient, who is positioned on an operating table in close proximity to the robotic unit. The surgeon controls the da Vinci System using his or her hands, via “master” instruments at the console, and the system seamlessly translates the surgeon’s hand, wrist and finger movements into precise, real-time movements of surgical instruments inserted into the patient’s body via small incisions. The EndoWrist instruments attached to the arms of the patient-side cart bend and rotate with wide range of high-precision movements (seven degrees of motion), which is even greater than the human wrist, and with the hand tremor filter. The robotic arms move around fixed pivot points to minimize stress on the thoracic wall during manipulations. The stereo-endoscope positioned in one of the robot arms allowed the surgeon to have a magnified, high-definition and three-dimensional view of the operating field via a console binoculars. The console also has foot pedals that allow the surgeon to engage and disengage different instrument arms, to reposition the console master controls without moving the instrument inside the patient, and to activate electric cautery.
With the introduction in 2014 of the last generation system (da Vinci Xi), some technical issues have been improved. First of all, a simpler docking due to a better maneuverability of the cart, improved by “port placement” menu and laser guidance in addition to the improved design of the arms, allows placement of the ports relatively close together reducing the risk of arm collision. Another important improvement is the possibility to use the EndoWrist Vessel Sealer as well as the EndoWrist Stapler (both 30 and 45 mm) entirely controlled by surgeon’s hands through the da Vinci Xi System, providing fully wristed articulation and SmartClamp feedback. Lastly, the thoracoscope has a lighter and digital end-mounted camera with autofocus for an improved crystal clear vision, and it could be placed on any robotic arms.
Robotic lobectomy approaches
During the last decade, different robotic approaches have been described on performing a lung resection ranging from the use of three or four robotic arms, utility incision or CO2 insufflation, and different port placement. To describe these approaches without being confusing, we decided to group them based on the use of utility incision (not completely portal lobectomy) or not [completely portal robotic lobectomy (CPRL)], highlighting the evolution and the changes of the robotic technique based on the surgeon’s experience over the years, as well as their short and long term results.
Not CPRL (with utility incision)
In 2006, Park (16) was the first to describe a 3-arm robotic approach with a non-rib-spreading utility incision, usually of 3–4 cm in the 4th or 5th intercostal space on the mid axillary line, and the use of two other trocars for the camera port (7th or 8th intercostal space at the posterior axillary line) and for the second instrument (just above the diaphragm posterior to the tip of the scapula), respectively (Figure 1). This robotic approach mimed their VATS lobectomy technique in term of patient and trocars position, other than anterior to posterior hilar vessels and bronchi dissection with the completion of the fissure performed last, just before the removal of the specimen; CO2 insufflation was not needed, the robot was positioned at the head of the patient (45° angle with respect to the long axis of the patient) and 30° camera was used. The results of Park preliminary study demonstrated that RATS was feasible and safe with 12% of conversion rate, higher than those reported in the contemporary largest series of VATS lobectomy but acceptable considering the early experience, with 4.5 days post-operatively length of stay, and no in-hospital or 30-day mortalities; besides, all patients had a R0 resection, with a median of four lymph node stations resected (16).
In 2010, Veronesi et al. (20) modified the 3-arm robotic approach described by Park introducing the use of a 4th robotic arm, positioned posteriorly in 7th intercostal space (auscultator triangle), to held the lung parenchyma in a fixed position, posteriorly, and have a better vision of the surgical field; 3 cm utility incision was performed at the 4th or 5th intercostal space, anteriorly, the camera port was positioned in 7th or 8th intercostal space on the mid-axillary line, and another port at the 7th or 8th intercostal space in the posterior axillary line (Figure 2). This 4-arm robotic lobectomy approach, always using 3-cm utility incision, was investigated by Veronesi on 54 patients between 2006 and 2008, and compare to the same number of patients having open lobectomy, using a propensity scores match showing a 13% of conversion rate, 4.5 days of post-operatively length of stay for RATS vs. 6 days for open surgery, and no difference in term of post-operative morbidity and mortality, and lymph nodes removed (20).
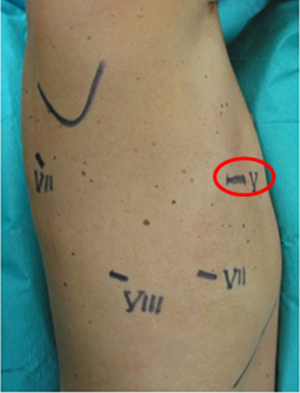
Since then, we always used the same 4-arm non-completely portal robotic approach performing 339 robotic anatomical lung resection (307 lobectomies, 29 segmentectomies and 3 pneumonectomies) with 6.5% of conversion rate, 5 days of postoperatively length of stay, no in-hospital or 30-day mortalities, and a median of five lymph node stations removed (21). The portal placement did not change with the type of resection or the side, except for left-sided operations where the camera port was placed more laterally to keep the heart out of the way, and with the different robotic system (S, SI and XI).
However, with the introduction of the new da Vinci XI robotic system in 2015, some technical changes were made: (I) the surgical cart was docked from the left side of the patient either for right or left thoracic procedures taking advantage of the technical innovation of this new robotic system to displace the four arms correctly despite its side position; (II) the new system allowed us to work with a minimum distance of 4 cm between the ports, without respecting a particular distance between the ports; (III) the camera could be moved between two different ports allowing a better view; (IV) the use of the EndoWrist stapler (30 vascular and 30 or 45 parenchyma) which could be placed through a 12-mm port (for inferior lobectomy and segmentectomy it was placed alternatively at the utility thoracotomy or posterior axillary line; for the upper lobectomy, it was placed at the posterior axillary line), as previously reported (22).
In this group of not completely portal lobectomy we included also the hybrid technique described by Gharagozloo et al. in 2008 (23). This approach involved the use of the Robot for the hilar and mediastinal dissection (vessels, bronchi and lymph nodes) and the use of VATS technique (when the robot is removed, and the surgeon is back to the operating table) for the vascular, parenchymal, and bronchial division; usually three 2-cm incisions were performed using a non-trocar technique, other than an additional 1 to 2-cm incision anteriorly for the endoscopic retractor and the chest tube at the end of the surgery (23). The author published 100 consecutive cases in 2009 showing no conversion rate, a median hospitalization of 4 days, 21% of post-operative morbidity with 3 post-operative death (3%) during the first 20 cases, (not due to the robotic technique but to a poor lung function of the patients), and concluded that robot assistance may facilitated nodal and vascular dissection during VATS lobectomy, even if increasing the complexity and the length of the procedure (24).
Why utility incision?
- To extract the specimens such as lymph nodes during the lymphadenectomy or the lung at the end of the surgery;
- To introduce small gauzes and suction instrument in case of bleeding;
- To palpate the nodule in case of wedge resection performed before lobectomy.
CPRL
Ninan and Dylewski in 2010 (25) reported the effectiveness of a completely portal robotic lobectomy using 3 arms (CPRL-3) without the need of utility incision. Robotic camera port for a 0° camera was placed in the 5th or 6th intercostal space along the major pulmonary fissure and two other ports were then placed in the same intercostal space anteriorly and posteriorly, to reduce intercostal neurovascular injuries. An utility 7-mm port was inserted anteriorly at the tip of the 11th rib on the anterior abdominal wall, and tunneled over the top of the 10th rib to place a 5-mm camera for the initial exploration of the thoracic cavity and for guiding the port placement; during surgery the utility access was used for suctioning and passages of staplers, and at the end of the surgery for removing the specimen, enlarging it to 3–4 cm (Figure 3). CO2 insufflation was used to move the diaphragm inferiorly facilitating the use of the utility access. In 2011, Dylewsky described 200 consecutive patients underwent anatomical lung resection using this completely portal robotic approach showing the safeness and feasibility of this technique: only 3 (1.5%) conversion to thoracotomy, a median length of hospital stay of 3 days, a 60-day mortality and morbidity of 2% and 26%, respectively; though, the number of lymph node or the lymph node station removed was not reported (26).
In 2011, Cerfolio et al. (27) described a new CPRL with 4 robotic arms technique (CPRL-4). In this new approach, all the four ports were placed along the 7th intercostal space between mid-axillary line, or as anteriorly as possible, and 2–3 cm lateral to the spinous process of the vertebral bodies always keeping 9–10 cm of distance between the ports; an access port was then place 2–3 ribs lower to give more working space to the assistant surgeon (Figure 4). Even in this CPRL-4, CO2 insufflation and 0° camera were used. Cerfolio demonstrated that the new CPRL-4 was safe and allowed R0 resection with complete lymph node removal (median of 5 N2, 3 N1 nodal stations, 17 lymph nodes removed). CPRL-4 was associated with lower post-operative morbidity and mortality, shorter hospital length of stay, and a better quality of life than rib-sparing thoracotomy, with a conversion rate of 12% (13/106) (27).

Why CO2 insufflation?
- To move the diaphragm inferiorly, creating more working space for the assistant through utility port;
- To facilitate the hilar dissection unsticking the tissue with the CO2 pressure;
- To squeeze the lung in case of incomplete pulmonary exclusion.
Oncologic outcome
One of the major criticisms of minimally invasive surgery is the inadequate mediastinal lymph node dissection compared to open surgery. Concern over inferior oncologic outcomes has contributed to the slow adoption of minimally invasive surgery techniques. However, the literature has already demonstrated that robotic pulmonary resection is oncological safe, allowing excellent lymph node removal (28-31). In particular, the 3D vision and the wide range of high-precision movements, even greater than the human wrist, are crucial in performing lymphadenectomy, allowing an excellent hilar and mediastinal lymph node dissection. Although different studies have demonstrated that RATS is associated with reduced mortality, shorter hospital stay, and fewer overall complications compare to open surgery, only few studies have evaluated oncological outcomes (28-31).
In literature, the median number of lymph nodes harvested by RATS range between 13.9 to 18 (28-32), compare to a median of 16 harvested by VATS (32) and 14.7 resected in open surgery (33).
Park et al. in 2012 (28) published a robotic multicenter experience showing an overall pathologic lymph node upstaging of 24% (18% N1 upstaging and 6% N2 upstaging), with similar results compare to the larger open series (14.3–24.6%) and better than conventional VATS (10.6–11.9%) (33-35). More recently, Toosi et al. (29) confirmed that RATS allowed an adequate lymphadenectomy with detection of occult lymph node metastatic disease, with significant upstaging (14.8%) and equal oncologic outcomes compare to open radical lymphadenectomy. In a multicenter study published in 2017, Cerfolio et al. (31) showed a median number of lymph nodes resected of 13 (5 N2 stations and 1 N1), with a cumulative incidence of local recurrence in the ipsilateral operated chest of 3% only.
Even in our experience, the overall median number of lymph node stations removed was five, with a median number of 15 lymph nodes harvested and an overall pathological lymph node upstaging of 17.6% (21). This highlights the excellent lymphadenectomy performed by robotic surgery which is comparable to open surgery and even better than VATS.
Cerfolio et al. (31) showed excellent 5-year stage-specific survival [83% for stage IA NSCLC, 77% for the stage IB, 68% for stage IIA, 70% for IIB, 62% for stage IIIA (N2 disease, 73%), and 31% for stage IIIB] similar to the data previously published in literature (28,29). Besides, Toosi et al. highlighted as patients pathologically staged with robotic surgery had a better stage-specific survival at the earlier stages compared with clinical stage suggesting that staging was significantly improved with robotic lymphadenectomy (29).
Conclusions
Besides the well-known short-term outcomes showing very low morbidity and mortality rates, it is becoming more clear as the adequate assesses of lymph node stations performed by RATS could lead to excellent and promising oncologic results. However, robotic surgery has still some limitations, including increased costs, absence of a tactile feedback, and the need for specialized equipment and training; beside, longer follow-up is still needed to have a correct vision of the long-term outcome.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Shanghai Chest for the series “Minimally Invasive Thoracic Oncological Surgery”. The article has undergone external peer review
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.03.07). The series “Minimally Invasive Thoracic Oncological Surgery” was commissioned by the editorial office without any funding or sponsorship. DG served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Shanghai Chest from Jul 2017 to Jun 2019. LS served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Shanghai Chest from Jul 2017 to Jun 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive summary: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7-37S.
- Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients a case-control study. Ann Thorac Surg 1999;68:194-200. [Crossref] [PubMed]
- Hoksch B, Ablassmaier B, Walter M, et al. Complication rate after thoracoscopic and conventional lobectomy. Zentralbl Chir 2003;128:106-10. [Crossref] [PubMed]
- Nakata M, Saeki H, Yokoyama N, et al. Pulmonary function after lobectomy video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2000;70:938-41. [Crossref] [PubMed]
- Nomori H, Ohtsuka T, Horio H, et al. Difference in the impairment of vital capacity and 6-minute walking after a lobectomy performed by thoracoscopic surgery, an anterior limited thoracotomy, an antero-axillary thoracotomy, and a posterolateral thoracotomy. Surg Today 2003;33:7-12. [Crossref] [PubMed]
- Yim AP, Wan S, Lee TW, et al. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg 2000;70:243-7. [Crossref] [PubMed]
- Li WW, Lee RL, Lee TW, et al. The impact of thoracic surgical access on early shoulder function video-assisted thoracic surgery versus posterolateral thoracotomy. Eur J Cardiothorac Surg 2003;23:390-6. [Crossref] [PubMed]
- McKenna RJ Jr, Wolf RK, Brenner M, et al. Is VATS lobectomy an adequate cancer operation? Ann Thorac Surg 1998;66:1903-8. [Crossref] [PubMed]
- Leschber G, Holinka G, Linder A. Video-assisted mediastinoscopic lymphadenectomy (VAMLA)—a method for systematic mediastinal lymph node dissection. Eur J Cardiothorac Surg 2003;24:192-5. [Crossref] [PubMed]
- Yim AP, Landreneau RJ, Izzat MB, et al. Is video-assisted thoracoscopic lobectomy a unified approach? Ann Thorac Surg 1998;66:1155-8. [Crossref] [PubMed]
- Daniels LJ, Balderson SS, Onaitis MW, et al. Thoracoscopic lobectomy a safe and effective strategy for patients with stage I lung cancer. Ann Thorac Surg 2002;74:860-4. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted Thoracic Surgery Lobectomy: Experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Whitson BA, D'Cunha J, Andre RS, et al. Thoracoscopic versus thoracotomy approaches to lobectomy: differential impairment of cellular immunity. Ann Thorac Surg 2008;86:1735-44. [Crossref] [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience withrobotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Giulianotti PC, Buchs NC, Caravaglios G, et al. Robot-assisted lung resection: outcomes and technical details. Interact Cardiovasc Thorac Surg 2010;11:388-92. [Crossref] [PubMed]
- Melfi FM, Mussi A. Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin 2008;18:289-95. vi-vii. [Crossref] [PubMed]
- Paul S, Jalbert J, Isaacs AJ, et al. Comparative effectiveness of robotic-assisted vs thoracoscopic lobectomy. Chest 2014;146:1505-12. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Casiraghi M, Galetta D, Borri A, et al. Ten years’ experience in robotic thoracic surgery for early stage lung cancer. Thorac Cardiovasc Surg 2018; In press.
- Galetta D, Casiraghi M, Pardolesi A, et al. New stapling devices in robotic surgery. J Vis Surg 2017;3:45. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B. Robot-assisted thoracoscopic lobectomy for early-stage lung cancer. Ann Thorac Surg 2008;85:1880-5; discussion 1885-6.
- Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 cases. Ann Thorac Surg 2009;88:380-4. [Crossref] [PubMed]
- Ninan M, Dylewski MR. Total port-access robot-assisted pulmonary lobectomy without utility thoracotomy. Eur J Cardiothorac Surg 2010;38:231-2. [Crossref] [PubMed]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Toosi K, Velez-Cubian FO, Glover J, et al. Upstaging and survival after robotic-assisted thoracoscopic lobectomy for non-small cell lung cancer. Surgery 2016;160:1211-8. [Crossref] [PubMed]
- Nasir BS, Bryant AS, Minnich DJ, et al. Performing Robotic Lobectomy and Segmentectomy: Cost, Profitability, and Outcomes. Ann Thorac Surg 2014;98:203-8; discussion 208-9. [Crossref] [PubMed]
- Cerfolio RJ, Ghanim AF, Dylewski M, et al. The long-term survival of robotic lobectomy for non-small cell lung cancer: A multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. [Crossref] [PubMed]
- Lee BE, Korst RJ, Kletsman E, et al. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg 2014;147:724-9. [Crossref] [PubMed]
- Merritt RE, Hoang CD, Shrager JB. Lymph node evaluation achieved by open lobectomy compared with thoracoscopic lobectomy for N0 lung cancer. Ann Thorac Surg 2013;96:1171-7. [Crossref] [PubMed]
- Licht PB, Jørgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg 2013;96:943-9; discussion 949-50. [Crossref] [PubMed]
- Osarogiagbon RU, Allen JW, Farooq A, et al. Objective review of mediastinal lymph node examination in a lung cancer resection cohort. J Thorac Oncol 2012;7:390-6. [Crossref] [PubMed]
Cite this article as: Casiraghi M, Galetta D, Spaggiari L. Robotic assisted lobectomy and lymphadenectomy “different approaches”. Shanghai Chest 2018;2:17.




