Minimally invasive esophagectomy: the current state of affairs
“The cleaner and gentler, the act of operation, the less pain the patient suffers, the smoother and quicker the convalescence, the more exquisite his healed wound, the happier his memory of the whole incident.”—Lord Moynihan (1920) (1).
Introduction
Esophageal cancer affects almost 460,000 individuals annually worldwide and the incidence continues to rise (2,3). While previously considered a highly lethal disease, improvements in medical and surgical therapies have seen an increase in overall 5-year overall survival (OS) from 5.1% to 18.8% over the past four decades (4,5). Surgical resection, either alone for early tumors or following neoadjuvant therapy for locoregional disease, remains the mainstay of curative treatment. Esophagectomy is considered one of the more technically challenging surgical procedures; however, perioperative outcomes are improving. Recent data from the Society of Thoracic Surgeons database reported a major complication rate of 33.1% and operative mortality rate of 3.1% (6). These rates are notable in comparison to complication rates of over 50% and death rate of 13.7% reported less than 15 years prior (7).
Surgical treatment has been demonstrated to be key in improving survival in patients with esophageal cancer (8). In spite of these findings, over 70% of eligible patients are not recommended to undergo surgical resection and up to 35% may not receive any treatment at all (9). Surgery for esophageal cancer is underutilized, and among other reasons, this may be largely resulting from concern over postoperative outcomes (10). Increased emphasis on improving postoperative outcomes and quality of life in conjunction with evolving surgical technologies has led to the evolution of minimally invasive surgical techniques. By reducing the “invasiveness” of open surgery, potential benefits of the minimally invasive technique may include reduced incision size and tissue trauma, better visualization of the surgical field and less postoperative complications resulting in improved patient satisfaction and survival (11,12).
Minimally invasive surgery for colon cancer has long been established as feasible and oncologically safe (13). Buy-in increased rapidly, with rates increasing from 35% in 2006 to 51% in 2010 (14). Minimally invasive esophagectomy (MIE) is more technically challenging, and has had a relatively slower increase in use. As surgeons gain experience with MIE, there has been increased utilization of this approach. In 2012–2014, 33.9% of esophagectomies performed in the United States utilized a minimally invasive technique (6).
In MIE, thoracotomy and laparotomy are replaced with thoracoscopy and laparoscopy. First described in 1992 by Cuschieri et al. utilizing a hybrid laparotomy/thoracoscopy approach, MIE has undergone significant advancements (15,16). The major advantage of MIE is the potential for decreased incidence of postoperative pulmonary infection (17). This is significant as respiratory complications are the most common complication after this procedure, and cause of up to two-thirds of fatalities associated with postoperative morbidity (18,19). In this paper we provide an overview of the critical aspects of MIE and compare outcomes with open esophagectomy.
Surgical techniques
The currently available surgical approaches for resection in patients with esophageal cancer include transhiatal esophagectomy (THE) and transthoracic esophagectomy (TTE), including the Ivor Lewis and McKeown techniques. In this section we review the basic principles of these MIE procedures—with particular emphasis to the Ivor Lewis approach which we perform more commonly—and subsequently we discuss the associated outcomes and procedure selection.
Preoperative patient preparation for esophagectomy requires a multidisciplinary approach. Patients should undergo appropriate oncologic staging, with subsequent referral for neoadjuvant chemoradiation as clinically indicated. Operative risk assessment should be performed with subsequent patient optimization. Esophagectomy should ideally be performed between 4 to 8 weeks following completion of neoadjuvant therapy. Neoadjuvant therapy has not been demonstrated to increase risk of postoperative complications within this period; however, it may be appropriate to delay surgery beyond this period in patients who have not yet recovered from therapy (20,21). Patients should be nutritionally optimized and encouraged to stop smoking. The safety and feasibility of esophagectomy has been demonstrated in elderly patients; however, limited studies have evaluated MIE in the elderly (22-24). Regardless of age, patients with comorbidities should be referred to appropriate medical consultants to mitigate risk factors for poor outcome postoperatively. Postoperative pulmonary complications are common after esophagectomy. Preoperative respiratory rehabilitation may reduce incidence of such events and should be considered in high risk patients (25).
In THE, an abdominal incision is used both to mobilize the stomach and mediastinal esophagus via the diaphragmatic hiatus. The proximal esophagus is then mobilized through a cervical incision and the anastomosis is performed in the neck. By incorporating a thoracic incision, TTE enables further mediastinal lymphadenectomy with an anastomosis in either the neck (McKeown) or chest (Ivor Lewis). We prefer a transthoracic approach for patients with cancer and more commonly perform an intrathoracic anastomosis since the majority of patients in the US present with distal esophageal adenocarcinoma.
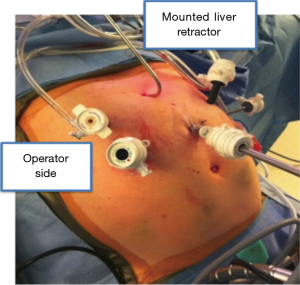
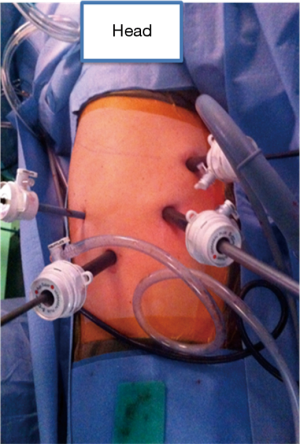
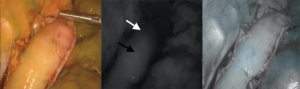
The abdominal portion of the procedure begins with the patient in a supine position with the surgeon standing on the right side of the patient. Upon entering the abdomen under direct vision in the left subcostal location, additional ports are placed in a similar configuration to a Nissen fundoplication (Figure 1). A staging laparoscopy is performed to evaluate for the presence of peritoneal metastasis. Laparoscopic ultrasound may be used to rule out liver involvement. The resection is begun with division of the lesser omentum and lymphadenectomy is performed along the celiac artery and its branches. The left gastric artery is divided using an endovascular stapler. Next, the gastrocolic ligament is divided with care to preserve the right gastroepiploic artery. The short gastric arteries are divided to free the greater curvature. The gastric fundus is freed from the diaphragmatic attachments and the esophagus is mobilized at the hiatus. A transhiatal dissection is then performed to mobilize the distal esophagus as up in the mediastinum as possible. The paracardial lymph nodes, which are difficult to reach via the thoracoscopic approach, are also removed. This maneuver often requires opening of the pleura, and as such it is performed toward the end of the abdominal phase due to potential for hemodynamic instability. Adjunct procedures, including a feeding jejunostomy and pyloric drainage (we prefer the use of Botox), are then performed. The conduit is then formed by tubularization of the stomach with serial firings of the endostapler from the lesser curvature to the fundus. Attention is then turned to the chest for thoracoscopic mobilization via right posterolateral thoracoscopy performed in the left lateral decubitus position. After port placement (Figure 2), the dissection is initiated with division of the mediastinal pleura both anteriorly and posteriorly to the esophagus. The dissection extends from the level of the azygous vein to the previous abdominal distal dissection. Care is taken to remain wide enough to include the periesophageal lymph nodes and create a wide radial margin while avoiding the thoracic duct, aorta and airways. A subcarinal lymphadenectomy is performed. The azygous vein is then divided and the esophagus proximal to it is prepared for anastomosis. The conduit is delivered into the chest and perfusion is assessed using fluorescence imaging (Figure 3). A suitable location is selected for creation of the anastomosis and it is then created in a stapled technique.
In the McKeown technique, the procedure begins with thoracic dissection similar to that in Ivor Lewis. Prior to beginning, a thoracoscopy is performed to evaluate for resectability and local invasion. En block dissection of the esophagus as well as mediastinal and upper abdominal lymphadenectomy is performed. The abdomen is then explored to rule out metastatic disease, and the stomach is mobilized as previously described. Finally, a cervical anastomosis is performed via a left neck incision.
In the THE approach the abdominal phase of the procedure is similar but extensive transdiaphragmatic dissection is required in order to mobilize the esophagus completely. The esophagus may be stripped without any periesophageal tissue which can compromise the radial margin of resection and thoracic lymphadenectomy. The cervical dissection is then performed through a left neck incision. The cervical esophagus is bluntly dissected distally to the proximal extent of the previous mediastinal dissection. The esophagus is transected in the low neck and extracted. The gastric conduit is then pulled up and a cervical anastomosis is performed either via a stapled or hand-sewn approach.
In each stage of these procedures, there are specific anatomic pitfalls that must be considered. In the abdominal phase, the celiac lymphadenectomy poses a significant risk of injury to the branches of the celiac axis, aorta, inferior vena cava and pancreas. Gastric mobilization must proceed cautiously; the right gastroepiploic artery arcade is the sole blood supply of the conduit. Excess manipulation of the stomach may damage vital submucosal vascular channels vital for perfusion of the proximal extent of the conduit. Care must be taken to ensure adequate division of the gastrocolic ligament in order to minimize the risk of paraconduit hernia formation. Finally, there is a risk of pneumothorax and hypotension with inadvertent entry into the pleural spaces during transhiatal dissection.
In the thoracic phase, it is important to avoid injury to the aorta or aorta-esophageal branches and to the thoracic duct and its branches. Caution must be exercised when performing paratracheal dissection to avoid injury to the recurrent laryngeal nerves. Additionally, there is a risk of perforation of the membranous airway with subcarinal lymphadenectomy. Finally, the orientation of the conduit should be noted as it is delivered into the chest. Performance of the anastomosis under tension will predispose to a leak. Caution should be exercised during cervical dissection to avoid injury to the recurrent laryngeal nerve.
Surgical outcomes
Proponents of MIE believe this approach will improve postoperative morbidity and quality of life without risking negative oncologic outcomes. There is increasing evidence that MIE is feasible and oncologically safe; however, the current literature remains controversial with respect to surgical outcomes. The decision of which operative approach (open vs. MIE) and technique (THE vs. TTE) to pursue may be dictated by tumor location and surgeon preference.
THE was proposed as an alternative surgical technique with the intent of reducing morbidity after esophagectomy by avoiding thoracotomy (26). Decreased rates of pneumonia and ventilator dependence have been reported but this has not been associated with a difference in mortality (27). Furthermore, THE is limited by poor visualization of the intrathoracic esophagus and limited access to mediastinal lymph nodes (28). While a survival advantage attributed to TTE has yet to be demonstrated in a large study, selected smaller reports have demonstrated a benefit with extended resection for locoregional disease (29,30).
Laparoscopic THE was first described by DePaula et al. in 1995 (31). As in the open approach, transhiatal MIE is associated with reduced pulmonary morbidity (32). In one of the largest studies comparing laparoscopic and open THE, MIE was associated with reduced length of hospital and intensive care unit (ICU) stay but demonstrated similar operative time and morbidity (32). Subsequently, a 2016 Cochrane Review comparing open versus laparoscopic THE demonstrated a reduced overall complication rate (62.3% vs. 39.9%; 95% CI, 0.48–0.86) and shorter median length of stay (33). A possible benefit to laparoscopic THE is the magnification offered by the laparoscope possibly improving visualization during mediastinal dissection (34).
The prevalence of adenocarcinoma of the esophagus and esophagogastric junction (EGJ) is highest in the Western world resulting in predominant use of the Ivor Lewis approach in this region (35). Advantages of the Ivor Lewis esophagectomy include direct visualization of the esophagus with excellent access for extensive mediastinal nodal dissection. Avoidance of cervical dissection and anastomosis is associated with reduced incidence of recurrent laryngeal nerve injury and subsequent aspiration risk (28,36). Furthermore, a more extensive gastric resection is made possible. The anastomosis can be performed using a segment of stomach with richer vascular supply resulting in reduced rates of anastomotic leak (37,38).
Total minimally invasive Ivor Lewis was first described in 1999 by Watson et al. using laparoscopic hand-assisted gastric mobilization and right thoracoscopy for esophageal dissection with an intrathoracic handsewn anastomosis (39). Subsequent improvements in operative technique led to favored use of a circular stapled anastomosis with introduction of the anvil through a transthoracic or transoral route (40). Stapled anastomosis has been demonstrated to result in less postoperative dysphagia and stricture (41). In a recent comparison of open esophagectomy and minimally invasive Ivor Lewis, no difference in operative time or cardiac complications was found; however, the authors found that MIE was associated with significantly reduced respiratory complications, intraoperative estimated blood loss (EBL) and intravenous fluid administration, and ICU and hospital LOS (18). The respiratory benefits were similarly demonstrated in another study (42). In contrast, Noble et al. similarly found reduced EBL but failed to observe a difference in pulmonary complications or LOS (43). While these results suggest equivalence, it is possible that further studies may confirm the observed reduction in pulmonary complications.
The three field esophagectomy was first described by McKeown in 1976; in this approach, a thoracotomy, laparotomy and cervical incision are performed in order to resect the esophagus and create a cervical anastomosis (44). A fundamental advantage of this approach is that it enables better proximal margins for patients with squamous tumors of the upper esophagus. Furthermore, the higher position of the cervical anastomosis may lead to a decrease in the effect of positive intra-abdominal pressure on the stomach resulting in a reduced rate of reflux esophagitis (45,46). Furthermore, there is decreased morbidity with a cervical anastomotic leak and clinical management in the case of a leak may be easier given the location (47). McKeown esophagectomy may be indicated in cases of high and mid-esophageal tumors, extended long-segment Barrett’s esophagus, and complex benign disorders (48).
Luketich et al. first reported on the feasibility of 3-hole MIE in 1998 (49). In a subsequent series of 222 patients, the authors demonstrated the procedure to be safe with low rates of pneumonia in comparison to other open series at the time (7.7%) (48,50). Further demonstration of the safety of this procedure was presented in a recent comparison of open and minimally invasive McKeown esophagectomies which showed comparable postoperative morbidity and mortality with similar oncologic outcomes (51).
In a recent meta-analysis of 1,681 patients undergoing total MIE, the results of Ivor Lewis and McKeown were compared (52). The authors found reduced incidence of recurrent laryngeal nerve trauma, LOS and EBL with Ivor Lewis. The Ivor Lewis procedure has shorter operative time with reduced risk of leak and vocal injury; however, it is a technically more challenging procedure and if a complication does occur then the patient may have a more complex course (48).
There have been a large number of studies published comparing open and MIE that have demonstrated inconclusive findings on the impact of MIE on surgical outcomes. Meta-analyses have suggested that MIE may lead to reduced morbidity with comparable mortality; however, systematic reviews have demonstrated equivocal results (34). The origin of the discrepancy in findings in the literature may be multifactorial. One possible explanation is that MIE is an especially technically challenging procedure and ation in surgeon skill levels may lead to the difference in reported results. MIE requires a steep learning curve to master the procedure (up to 50 cases) (53).
Several meta-analyses have been published comparing outcomes of open and MIE. Nagpal et al. demonstrated shorter LOS and reduced respiratory and overall morbidity with MIE (54). Other studies demonstrated longer operative time, reduced in-hospital mortality, and better quality of life 3 months postoperatively with MIE (55-57). In contrast, another meta-analysis failed to demonstrate a difference in pulmonary or overall complications (58). A recent propensity score matched analysis of 1,727 patients demonstrated a higher incidence of anastomotic leak and reinterventions after MIE (59). In the TIME randomized control trial (Traditional Invasive vs. MIE), the authors found that incidence of postoperative pulmonary infection was significantly lower in MIE (12% vs. 34%, P=0.005) (60). The benefits of the hybrid surgical approach have also been considered. In a subgroup comparison of thoracoscopy/laparotomy vs. open TTE, Biere et al. found lower rates of anastomotic leak in the MIE group while another study demonstrated no difference (58,61). Although the findings are contrasting at times, there is enough evidence to suggest that surgical outcomes are at least equivalent if not better with MIE.
Prone positioning has been described for both TTE approaches (11). The proponents of this technique report several advantages of thoracoscopic mobilization of the esophagus in the prone position including use of a single-lumen endotracheal tube; decreased lung, bronchial and tracheal injury; decreased anesthesia time; excellent operative exposure; and improved surgeon ergonomics. The reduction in traumatic insult to the lung and shorter operative time theoretically yields improved postoperative respiratory function (62). The major criticism of this technique is that repositioning may be necessary in the event that conversion is required. Additionally, a cervical anastomosis is required with this approach. A systematic review of 723 patients demonstrated increased mediastinal nodal harvest and reduced pulmonary complications and EBL with prone positioning (63).
The role of pyloric drainage during esophagectomy remains unclear. The proposed benefit includes decreased risk of aspiration pneumonia by preventing gastric outlet obstruction (GOO); however, obstruction is rare, and these procedures may predispose patients to dumping and bile reflux later in the postoperative period. A meta-analysis of 553 patients concluded that pyloric drainage reduced occurrence of early postoperative GOO with little effect on other outcomes (64).
Surgeon experience with MIE is increasing; however, pyloric drainage in this setting may be more technically challenging than in the open approach. Intrapyloric injection of botulinum toxin, first proposed in 2007, has become an increasingly popular alternative to surgical drainage procedures (pyloroplasty/pyloromyotomy) (65). Data are lacking to demonstrate whether one technique is safest in MIE. A recently published comparison of 146 patients who underwent MIE demonstrated no difference between surgical drainage and botulinum injection; however, when adjusting for the MIE approach, it was found that botulinum injection did result in the need for fewer postoperative interventions (66). Our current practice is to use botulinum toxin.
Oncologic outcomes
Heterogeneity in technique for open and MIE surgery, and differences in multimodality therapy protocols, make the evaluation of oncological outcomes challenging. A meta-analysis from 2012 of 1,212 patients found no difference in survival rates for MIE and open esophagectomy (67). Similar outcomes have been demonstrated in several other studies (42,68). In recently published follow-up data from the TIME trial, no difference was demonstrated in disease-free and overall 3-year survival between open and minimally invasive surgery patients (69). While several studies have demonstrated higher lymph node harvest in patients who undergo MIE, others have found no difference (42,68,70). It is possible that utilizing the thoracoscopic or laparoscopic camera during lymphadenectomy improves visualization. Further controversy surrounds the question of adequacy of resected margins. Several studies have demonstrated no difference in resection margins between groups (18,42,43,71). Current evidence supports the safety and feasibility of use of MIE for esophageal cancer.
Cost analysis
The cost effectiveness of MIE is controversial and generalizations are difficult given limited publications on the subject and differences in healthcare costs between countries. Operative costs for minimally invasive surgery are higher than for open procedures; however, this may be offset by faster recovery time and shorter LOS. In a 2013 study from Canada, Lee et al. determined that MIE resulted in decreased costs and increased quality-adjusted life years (72). The authors further concluded that analyzing the costs of lost productivity and burden to caregivers would likely increase the cost-effectiveness of minimally invasive surgery. A European study from 2009 found higher operative costs, due to the use of specialized and disposable equipment, but lower inpatient costs yielding similar overall costs between minimally invasive and open Ivor Lewis procedures (73).
In the United States, two studies have evaluated the differences in cost between MIE and open surgery with conflicting results. In a comparison of MIE and open transhiatal and transthoracic procedure costs, Dhamija et al. found that hospitalization and intraoperative costs were higher than the open approach and that shorter LOS did not offset increased costs (74). In comparison Towe et al. reported that despite increased intraoperative costs associated with MIE, total hospital costs did not differ between groups (75). When controlling for surgical technique and preoperative comorbidities, an overall cost increase of $17,835 was associated with hospitalizations in which postoperative complications occurred. It is possible that use of enhanced recovery pathways (ERAS) after esophagectomy may improve safety and outcomes for all patients while reducing costs. One study of implementation of ERAS after esophagectomy yielded overall costs savings of €2013 per patient (76). Future studies should further assess the impact of ERAS on hospitalization costs after esophagectomy.
New innovations
Robot-assisted MIE (RAMIE) has been adapted by some institutions, but published data is very limited. Several small series suggest similar surgical safety when compared to MIE (77,78). Furthermore, RAMIE has been demonstrated to be oncologically effective with acceptable lymphadenectomy (79). Creation of the stapled anastomosis is one of the most technically challenging steps of MIE. The improved suturing capabilities with the use of the robot may facilitate the creation of the anastomosis during RAMIE (77). The ROBOT trial is the first randomized controlled trial to compare the safety of robot-assisted minimally invasive thoraco-laparoscopic esophagectomy to open esophagectomy and it is expected its results will demonstrate superiority of this approach with respect to postoperative morbidity (80).
Conclusions
The use of MIE has been increasing since it was first introduced. Although current data suggests it may be beneficial, the majority of esophagectomies are still performed open. MIE appears to be safe with lower morbidity and similar oncologic outcomes than open esophagectomy. We anticipate that as surgeons gain experience with the technique, the clear benefits with use of MIE will be widely reported.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lorenzo Spaggiari and Domenico Galetta) for the series “Minimally Invasive Thoracic Oncological Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.03.01). The series “Minimally Invasive Thoracic Oncological Surgery” was commissioned by the editorial office without any funding or sponsorship. DM reports grants from Intuitive, other from Urogen, other from Johnson and Johnson, other from Boston Scientific, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moynihan BG. The ritual of a surgical operation. Br J Surg 1920;8:27-35. [Crossref]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2006, National Cancer Institute. Available online: http://seer.cancer.gov/csr/1975_2006/
- National Cancer Institute. SEER Stat Fact Sheets: Esophageal Cancer. Available online: http://seer.cancer.gov/statfacts/html/esoph.html
- The Society of Thoracic Surgeons General Thoracic Surgery Database Task Force. The society of thoracic Surgeons composite score for evaluating esophagectomy for esophageal cancer. Ann Thorac Surg 2017;103:1661-7. [Crossref] [PubMed]
- McCulloch P, Ward J, Tekkis PP. for the ASCOT group of surgeons, on behalf of the British Oesophago-Gastric Cancer Group. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ 2003;327:1192-7. [Crossref] [PubMed]
- Abrams JA, Buono DL, Strauss J, et al. Esophagectomy compared to chemoradiation for early stage esophageal cancer in the elderly. Cancer 2009;115:4924-33. [Crossref] [PubMed]
- Molena D, Stem M, Blackford AL, et al. Esophageal Cancer Treatment Is Underutilized Among Elderly Patients in the USA. J Gastrointest Surg 2017;21:126-36. [Crossref] [PubMed]
- Dubecz A, Sepesi B, Salvador R, et al. Surgical resection for locoregional esophageal cancer is underutilized in the United Sttes. J Am Coll Surg 2010;211:754-61. [Crossref] [PubMed]
- Cuesta MA, van der Peet DL, Biere SS, et al. Thoracoscopic esophagectomy. In: Bonavina L, editor. Innovation in esophageal surgery, Milan: Springer-Verlag Italia, 2012:65-75.
- Mack MJ. Minimally invasive and robotic surgery. JAMA 2001;285:568-72. [Crossref] [PubMed]
- Sargent DJ, Wieand HS, Fleshman J, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-9. [Crossref] [PubMed]
- Yeo H, Niland J, Milne D, et al. Incidence of minimally invasive colorectal cancer surgery at National Comprehensive Cancer Network Centers. J Natl Cancer Inst 2014;107:362. [Crossref] [PubMed]
- Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracopscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed]
- Fabian T, Frederico JA. The impact of minimally invasive esophageal surgery. Surg Clin N Am 2017;97:763-70. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Sihag S, Wright CD, Wain JC, et al. Comparison of perioperative outcomes following open versus minimally invasive ivor lewis oesophagectomy at a single, high-volume centre. Eur J Cardiothorac Surg 2012;42:430-7. [Crossref] [PubMed]
- Dumont P, Wihlm JM, Hentz JG, et al. Respiratory complications after surgical treatment of esophageal cancer: a study of 309 patients according to the type of resection. Eur J Cardiothorac Surg 1995;9:539-43. [Crossref] [PubMed]
- Kumagai K, Rouvelas I, Tsai JA, et al. Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. Br J Surg 2014;101:321-38. [Crossref] [PubMed]
- Kim JY, Correa AM, Vaporciyan AA, et al. Does the timing of esophagectomy after chemoradiation affect outcome? Ann Thorac Surg 2012;93:207-12; discussion 212-3. [Crossref] [PubMed]
- Perry Y, Fernando HC, Buenaventura PO, et al. Minimally invasive esophagectomy in the elderly. JSLS 2002;6:299-304. [PubMed]
- Ruol A, Portale G, Zaninotto G, et al. Results of esophagectomy for esophageal cancer in elderly patients: age has little influence on outcome and survival. J Thorac Cardiovasc Surg 2007;133:1186-92. [Crossref] [PubMed]
- Puntambekar S, Kenawadekar R, Pandit A, et al. Minimally invasive esophagectomy in the elderly. Indian J Surg Oncol 2013;4:326-31. [Crossref] [PubMed]
- Inoue J, Ono R, Makiura D, et al. Prevention of postoperative pulmonary complications through intensive preoperative respiratory rehabilitation in patients with esophageal cancer. Dis Esophagus 2013;26:68-74. [Crossref] [PubMed]
- Atkins BZ, D’Amico TA. Respiratory complications after esophagectomy. Thorac Surg Clin 2006;16:35-48. [Crossref] [PubMed]
- Bhayani NH, Gupta A, Dunst CM, et al. Esophagectomies with thoracic incisions carry increased pulmonary morbidity. JAMA Surg 2013;148:733-8. [Crossref] [PubMed]
- Huang L, Onaitis M. Minimally invasive and robotic Ivor lewis esophagectomy. J Thorac Dis 2014;6:S314-21. [PubMed]
- Johansson J, DeMeester TR, Hagen JA, et al. En bloc vs transhiatal esophagectomy for stage T3 N1 adenocarcinoma of the distal esophagus. Arch Surg 2004;139:627-31. [Crossref] [PubMed]
- Rizzetto C, DeMeester SR, Hagen JA, et al. En block esopahgectomy reduces local recurrence and improves survival compared with transhiatal resection after neoadjuvant therapy for esophageal adenocarcinoma. J THorac Cardiovasc Surg 2008;135:1228-36. [Crossref] [PubMed]
- DePaula AL, Hashiba K, Ferreira EA, et al. Laparoscopic transhiatal esophagectomy with esophagogastroplasty. Surg Laparosc Endosc 1995;5:1-5. [PubMed]
- Maas KW, Biere SS, Scheepers JJ, et al. Laparoscopic versus open transhiatal esophagectomy for distal and esophageal junction cancer. Rev Esp Enferm Dig 2012;104:197-202. [Crossref] [PubMed]
- Gurusamy KS, Pallari E, Midya S, et al. Laparoscopic versus open transhiatal oesophagectomy for oesophageal cancer. Cochrane Database of Systematic Reviews 2016;3:CD011390 [PubMed]
- Cash JC, Zehetner J, Hedayati B, et al. Outcomes following laparoscopic transhiatal esophagectomy for esophageal cancer. Surg Endosc 2014;28:492-9. [Crossref] [PubMed]
- Rubenstein JH, Shaheen NJ. Epidemiology, diagnosis, and management of esophageal adenocarcinoma. Gastroenterology 2015;149:302-17.e1. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Zhai C, Liu Y, Li W, et al. A comparison of short-term outcomes between Ivor-Lewis and McKeown minimally invasive esophagectomy. J Thorac Dis 2015;7:2352-8. [PubMed]
- Biere SS, Maas KW, Cuesta MA, et al. Cervical or thoracic anastomosis after esophagectomy for cancer: A systematic review and meta-analysis. Dig Surg 2011;28:29-35. [Crossref] [PubMed]
- Watson DI, Davies N, Jamieson GG. Totally endoscopic Ivor Lewis esophagectomy. Surg Endosc 1999;13:293-7. [Crossref] [PubMed]
- Maas KW, Biere SS, Scheepers JJ, et al. Minimally invasive intrathoracic anastomosis after Ivor Lewis esophagectomy for cancer: a review of transoral or transthoracic use of staplers. Surg Endosc 2012;26:1795-802. [Crossref] [PubMed]
- Blackmon SH, Correa AM, Wynn B, et al. Propensity-matched analysis of three techniques for intrathoracic esophagogastric anastomosis. Ann Thorac Surg 2007;83:1805-13. [Crossref] [PubMed]
- Tapias LF, Mathisen DJ, Wright CD, et al. Outcomes with open and minimally invasive Ivor Lewis esophagectomy after neoadjuvant therapy. Ann Thorac Surg 2016;101:1097-103. [Crossref] [PubMed]
- Noble F, Kelly JJ, Bailey IS, et al. A prospective comparison of totally minimally invasive versus open ivor lewis esophagectomy. Dis Esophagus 2013;26:263-71. [Crossref] [PubMed]
- McKeown KC. Total three-stage oesophagectomy for cancer of the oesophagus. Br J Surg 1976;63:259-62. [Crossref] [PubMed]
- Chen KN. Managing complications I: leaks, strictures, emptying, reflux, chylothorax. J Thorac Dis 2014;6:S355-63. [PubMed]
- Shibuya S, Fukudo S, Shineha R, et al. High incidence of reflux esophagitis observed by routine endoscopicexamination after gastric pull-up esophagectomy. World J Surg 2003;27:580-3. [Crossref] [PubMed]
- D’Amico TA. Mckeown esophagogastrectomy. J Thorac Dis 2014;6:S322-4. [PubMed]
- Perry Y, Fernando HC. Three-field minimally invasive esophagectomy: Current results and technique. J Thorac Cardiovasc Surg 2012;144:S63-6. [Crossref] [PubMed]
- Luketich JD, Nguyen NT, Weigel T, et al. Minimally invasive approach to esophagectomy. JSLS 1998;2:243-7. [PubMed]
- Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: Outcomes in 222 patients. Ann Surg 2003;238:486-94. [PubMed]
- Chen B, Zhang B, Zhu C, et al. Modified McKeown minimally invasive esophagectomy for esophageal cancer: A 5-year retrospective study of 142 patients in a single institution. PLOS ONE 2013;8:e82428 [Crossref] [PubMed]
- van Workum F, Berkelmans GH, Klarenbeek BR, et al. McKeown or Ivor Lewis totally minimally invasive esophagectomy for cancer of the esophagus and gastroesophageal junction: systematic review and meta-analysis. J Thorac Dis 2017;9:S826-33. [Crossref] [PubMed]
- Tapias LF, Morse CR. Minimally invasive Ivor Lewis esophagectomy: Description of a learning Curve. J Am Coll Surg 2014;218:1130-40. [Crossref] [PubMed]
- Nagpal K, Ahmed K, Vats A, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 2010;24:1621-9. [Crossref] [PubMed]
- Xiong WL, Li R, Lei HK, et al. Comparison of outcomes between minimally invasive oesophagectomy and open oesophagectomy for oesophageal cancer. ANZ J Surg 2017;87:165-70. [Crossref] [PubMed]
- Zhou C, Zhang L, Wang H, et al. Superiority of minimally invasive oesophagectomy in reducing in-hospital mortality of patients with resectable oesophageal cancer: A meta-analysis. PLos ONE 2015;10:e0132889 [Crossref] [PubMed]
- Kauppila JH, Xie S, Johar A, et al. Meta-analysis of health-related quality of life after minimally invasive versus open oesophagectomy for oesophageal cancer. Br J Surg 2017;104:1131-40. [Crossref] [PubMed]
- Biere SS, Cuesta MA, van der Peet DL. Minimally invasive versus open esophagectomy for cancer: a systematic review and meta-analysis. Minerva Chir 2009;64:121-33. [PubMed]
- Seesing MF, Gisbertz SS, Goense L, et al. A propensity score matched analysis of open versus minimally invasive transthoracic esophagectomy in the Netherlands. Ann Surg 2017;266:839-46. [Crossref] [PubMed]
- Biere SSAY, Maas KW, Bonavina L, et al. Traditional invasive vs. minimally invasive esophagectomy: a multi-center, randomized trial (TIME-trial). BMC Surg 2011;11:2. [Crossref] [PubMed]
- Sgourakis G, Gockel I, Radtke A, et al. Minimally invasive versus open esophagectomy: meta-analysis of outcomes. Dig Dis Sci 2010;55:3031-40. [Crossref] [PubMed]
- Palanivelu C, Prakash A, Senthilkumar R, et al. Minimally invasive esophagectomy: Thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position – experience of 130 patients. J Am Coll Surg 2006;203:7-16. [Crossref] [PubMed]
- Markar SR, Wiggins T, Antonowicz S, et al. Minimally invasive esophagectomy: Lateral decubitus vs. prone positioning; systematic review and pooled analysis. Surg Oncol 2015;24:212-9. [Crossref] [PubMed]
- Urschel JD, Urschel DM, Miller JD, et al. A meta-analysis of randomized controlled trials of route of reconstruction after esophagectomy for cancer. Am J Surg 2001;182:470-5. [Crossref] [PubMed]
- Kent MS, Pennathur A, Fabian T, et al. A pilot study of botulinum toxin injection for the treatment of delayed gastric emptying following esophagectomy. Surg Endosc 2007;21:754-7. [Crossref] [PubMed]
- Giugliano DN, Berger AC, Meidl H, et al. Do intraoperative pyloric interventions predict the need for postoperative endoscopic interventions after minimally invasive esophagectomy? Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
- Dantoc M, Cox MR, Eslick GD. Evidence to support the use of minimally invasive esophagectomy for esophageal cancer: A meta-analysis. Arch Surg 2012;147:768-76. [Crossref] [PubMed]
- Dolan JP, Kaur T, Diggs BS, et al. Impact of comorbidity on outcomes and overall survival after open and minimally invasive esophagectomy for locally advanced esophageal cancer. Surg Endosc 2013;27:4094-103. [Crossref] [PubMed]
- Straatman J, van der Wielan N, Cuesta MA, et al. Minimally invasive versus open esophageal resection- Three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Yerokun BA, Zhifei S, Chi-Fu JY. Minimally invasive versus open esophagectomy for esophageal cancer: A population-based analysis. Ann Thorac Surg 2016;102:416-23. [Crossref] [PubMed]
- Palazzo F, Rosato EL, Chaudhary A, et al. Minimally invasive esophagectomy provides significant survival advantage compared with open or hybrid esophagecomy for patients with cancers of the esophagus and gastroesophageal junction. J Am Coll Surg 2015;220:672-9. [Crossref] [PubMed]
- Lee L, Sudarshan M, Li C, et al. Cost-effectiveness of minimally invasive versus open esophagectomy for esophageal cancer. Ann Surg Oncol 2013;20:3732-9. [Crossref] [PubMed]
- Parameswaran R, Veeramootoo D, Krishnadas R, et al. Comparative experience of open and minimally invasive esophagogastric resection. World J Surg 2009;33:1868-75. [Crossref] [PubMed]
- Dhamija A, Dhamija A, Hancock J, et al. Minimally invasive oesophagectomy more expensive than open despite shorter length of stay. Eur J Cardiothorac Surg 2014;45:904-9. [Crossref] [PubMed]
- Fu SJ, Ho VP, Ginsberg J, et al. Complications, not minimally invasive surgical technique, are associated with increased cost after esophagectomy. Minim Invasive Surg 2016;2016:7690632.
- Lee L, Li C, Robert N, et al. Economic impact of an enhanced recovery pathway for oesophagectomy. Br J Surg 2013;100:1326-34. [Crossref] [PubMed]
- Okusanya OT, Sarkaria IS, Hess NR, et al. Robotic assisted minimally invasive esophagectomy (RAMIE): the University of Pittsburgh Medical Center initial experience. Ann Cardiothorac Surg 2017;6:179-85. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Grosser R, et al. Attaining proficiency in robotic-assisted minimally invasive esophagectomy while maximizing safety during procedure development. Innovations 2016;11:268-73. [PubMed]
- van der Sluis PC, Ruurda JP, Verhage RJ, et al. Oncologic long-term results of robot-assisted minimally invasive thoraco-laparoscopic esophagectomy with two-field lymphadenectomy for esophageal cancer. Ann Surg Oncol 2015;S1350-6. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer, a randomized controlled trial (ROBOT trial). Trials 2012;13:230. [Crossref] [PubMed]
Cite this article as: Nobel TB, Barbetta A, Molena D. Minimally invasive esophagectomy: the current state of affairs. Shanghai Chest 2018;2:21.