Modern diagnostic and therapeutic interventional pulmonology in mesothelioma
Introduction
Mesothelioma is a relatively rare tumour, but incidence is increasing in view of the time lag of 30–50 years after exposure to asbestos. In the Western world, mesothelioma incidence is expected to be reaching a peak (1-3). However, the continued use of asbestos in the developing world means that worldwide mesothelioma incidence could continue to increase in coming years (3). More invasive procedure techniques were traditionally reserved for thoracic surgeons or interventional radiologists, however the use of ultrasound by general physicians has increased over the past few years, and with this came an associated expansion in the field of interventional pulmonology available for pleural disease including mesothelioma, although available interventions will vary geographically. This article reviews the recent diagnostic and therapeutic advances in interventional pulmonology for mesothelioma.
Diagnostics
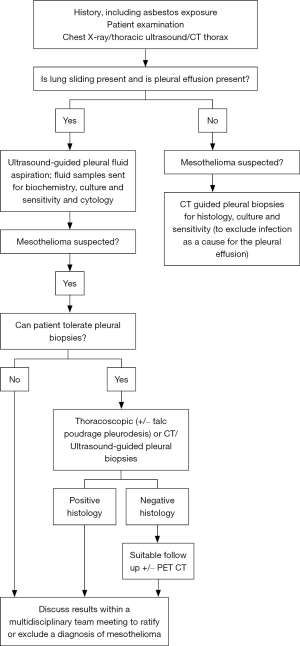
Obtaining a diagnosis of mesothelioma is important both clinically and medico-legally. It is essential to combine history, examination, radiology and pathology to reach a diagnosis of mesothelioma. It is also advised to discuss cases in detail and agree a diagnosis at a multidisciplinary team (MDT) meeting level, preferably in MDTs with significant experience of mesothelioma diagnosis.
The diagnosis of mesothelioma can be difficult, with some cases demonstrating atypical histological features, fibrosis rather than frank malignant features (desmoplastic), and biopsies may be challenging to achieve in some patients. High quality diagnosis therefore relies on a combination of radiological, clinical and pathological features, and one feature in isolation should not be used to make a diagnosis where possible.
Extensive research has been conducted on serum biomarkers to aid diagnosis, but only a few have shown any efficacy in the diagnostic process. Although a large number of studies have assessed potential and specific biomarkers in mesothelioma diagnosis, none has achieved the necessary high sensitivity and specificity to enter in to routine clinical practice, in addition to the lack of validation of promising results, and lack of specificity for mesothelioma (e.g., fibulin-3 and mesothelin) (4,5). One potential exception to this is carcinoembryonic antigen (CEA) which may prove useful as a negative marker (i.e., used to rule out mesothelioma) if cytological and histological analysis is inconclusive (3). Therefore, at present, histological diagnosis remains the preferred method of diagnosing mesothelioma. Figure 1 summarises the suggested diagnostic pathway for mesothelioma.
Pleural aspiration
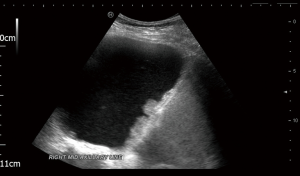
Given that pleural effusion is usually one of the first clinical signs of mesothelioma, pleural fluid cytology is often one of the first diagnostic tests to be carried out. Analysis of pleural fluid can help exclude other causes of pleural effusion (of which there are more than 60, and mesothelioma/malignancy is only one) and strengthen the suspicion of mesothelioma in the case of exudates with cytology suspicious for mesothelioma. The use of ultrasound to guide pleural fluid aspiration is increasingly being accepted as gold standard of practice (6). Apart from improving the safety profile of pleural procedures, ultrasound can give further information about the likelihood of underlying malignant pleural disease including mesothelioma by identifying pleural thickening and nodularity and distinguish malignant from benign pleural effusions with an overall sensitivity of 79% and specificity of 100% (7). It can also assess suitability for further pleural procedures including assessment of lung sliding to identify suitable candidates for thoracoscopy. Figure 2 shows an ultrasound view of diaphragmatic nodularity.
If clinical, radiological and cytological results support a diagnosis of mesothelioma, then this can be accepted as adequate to make a diagnosis of mesothelioma at a MDT meeting level in some cases where patients cannot tolerate pleural biopsies. However, pleural fluid cytology is reported to be up to 76% sensitive in specialist centres (1), and this is likely to be because the pre-test probability in cases seen in these centres is already high, and therefore this is not applicable to other centres. In fact, the pleural fluid cytology sensitivity for mesothelioma is usually quoted to be lower, with a definite diagnosis of mesothelioma made in 32.8% in one large case series (8). The differentiation of mesothelioma from other tumours such as adenocarcinoma, and further classification of mesothelioma into subtypes (epithelioid, biphasic and sarcomatoid), from cytology alone can be challenging. In addition, the identification of the subtype has significant implications for both treatment (for example, sarcomatoid mesothelioma is not conventionally considered responsive to standard chemotherapy) and prognosis (9). The histopathological subtype is important because, together with performance status, it is one of the few clinically significant prognostic factors (2). Furthermore, even with histological analysis, differentiation between mesothelioma and reactive changes in the pleura secondary to metastatic disease can be challenging (3). Therefore, adequate pleural biopsies are usually needed for accurate histological analysis, and the European Respiratory Society/European Society of Thoracic Surgeons (ERS/ESTS) task force and the European Society for Medical Oncology (ESMO) guidelines both recommend that pleural biopsy histology is required to confirm a diagnosis of mesothelioma (2,3).
Pleural biopsies
Patients with cytology negative pleural effusion, or with cytology suspicious of mesothelioma, should be followed up by tissue confirmation (2,3). This also allows confirmation of histological diagnosis, and in addition molecular and immunohistochemical analyses. Importantly for mesothelioma diagnosis and treatment, the majority of staging systems involve an assessment of the extension of the tumour in the pleura and muscle layers (3).
There are a number of potential pleural biopsy options open to the interventional pulmonologist as below:
Blind pleural biopsies
Blind pleural biopsies taken using “closed” biopsy needles (such as the Abram’s and Cope needles) in the absence of image guidance can make a diagnosis of mesothelioma, but only if there is sufficient material representative of the tumour (3). In malignant pleural effusions, blind pleural biopsies increase diagnostic yield above pleural fluid cytology only modestly, by 7–27% (10). Pleural fluid cytology and blind pleural biopsy have, in combination, a very poor diagnostic sensitivity for mesothelioma at 38.7% (11). Specific issues with non-image-guided closed pleural biopsy include the potential of not obtaining pleural tissue, and the patchy nature of malignant pleural disease which tends to preferentially occur in the inferior and lateral areas of the hemithorax, often lower than is usual to biopsy with a blind technique (12).
Image-guided pleural biopsies
Where available, image-guided biopsies are preferable to blind biopsies because of the increased diagnostic yield with image-guided procedures which target high value areas and are more likely to obtain pleural tissue. Image guidance helps to specifically target abnormal pleura, as opposed to blind pleural biopsies, which may sample normal areas of pleura in view of the patchy malignant infiltration. Real-time ultrasound or computed-tomography (CT) guidance increases the safety of the procedure, and also increases the sensitivity of cutting-needle biopsies, from 30% for pleural fluid cytology and non-image-guided Abram’s or Cope biopsies, to 86% with CT- or ultrasound-guided cutting-needle pleural biopsies (13). Pleural biopsies obtained using Abram’s needle with CT guidance resulted in a high sensitivity of 82.4% in a study of 150 patients with cytology negative exudative pleural effusions (14), and blind pleural biopsies were found to have 47% sensitivity for malignant pleural effusion compared to 87% for CT-guided biopsies in another study (15). However, image-guided pleural biopsies using Abram’s or Cope needles are still a reasonable option where thoracoscopy is not possible.
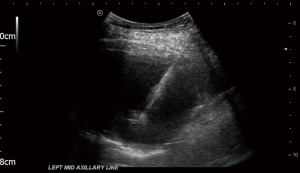
Physician led image-guided pleural biopsies are now being performed more frequently, a shift from what was once a solely radiologist’s domain. In patients who are frail, unable to tolerate lying in a lateral decubitus position for the duration of thoracoscopy, and for patients with a performance status >2, pleural biopsy under direct ultrasound guidance allows targeting of areas of interest, such as pleural thickening and nodularity. It can be an on-table alternative to thoracoscopy, should thoracoscopy not be deemed suitable (16). It has a diagnostic yield of about 94% when performed by respiratory physicians or by radiologists, and a similar diagnostic yield to CT-guided biopsies (16) and avoids the radiation exposure associated with CT guidance. Real-time direct ultrasound guidance enables the operator to ensure that samples were being taken from the area of pleural abnormality (16), and also allows compensation for respiratory movement in real-time. Ultrasound-assisted Tru-Cut needle pleural biopsies without real-time direct ultrasound observation of the needle have only a 66.7% sensitivity in cytology negative pleural effusions (14). Figure 3 shows a real-time ultrasound view of a biopsy needle entering the pleural space, with the tip of the needle within the pleural fluid. A diagnostic sensitivity of 89.7% was reported for thoracocentesis and ultrasound-assisted pleural biopsy in malignancy (17).
Thoracoscopy
CT-guided biopsies lack the ability to visualise the whole pleura, and thoracoscopy overcomes this, while avoiding the radiation exposure associated with CT. Medical thoracoscopy is recommended as the ideal diagnostic investigation for cytology negative exudates, especially if a diagnosis of mesothelioma is suspected (2,3) given that it combines a means of obtaining multiple larger pleural biopsies with a targeted sampling approach, and provides a diagnosis in >90% of cases. It has a high diagnostic sensitivity in malignant pleural effusion of 90.1–92.6%, which is similar to that in video-assisted thoracic surgery (VATS) (18). VATS still has a valid role in the diagnosis of pleural malignancy with a 90–95% diagnostic yield, similar to that for medical thoracoscopic pleural biopsies, however involves general anaesthetic, whereas medical thoracoscopy is performed under local anaesthetic and sedation. VATS is now often only considered following negative fluid cytology and closed biopsy histology, where strong suspicion of mesothelioma remains (19,20), and where a firm diagnosis will alter management.
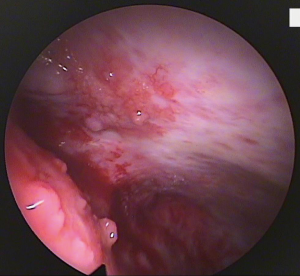
Thoracoscopy allows visually guided pleural biopsies targeting the visually abnormal parietal pleura, and the quality of tissue obtained is usually superior to that in closed biopsies, and this is important especially in complex diagnoses such as mesothelioma. It also allows minimally invasive complete visualisation of the pleura, documentation of the macroscopic appearance of pleural thickening, nodularity, lymphangitis, and involvement of the visceral pleura which upstages mesothelioma from T1a to T1b (2), and it also provides an opportunity to perform therapeutic interventions during the same procedure. Figure 4 shows the thoracoscopic view of nodular parietal and visceral pleura in mesothelioma, and Figure 5 shows the taking of biopsies of pleural nodules at thoracoscopy. Ultrasound helps guide the planning of the procedure by identifying the pleural effusion distribution, size, loculation and septation, the diaphragm position, and it can also confirm the presence of lung sliding and exclude significant pleural adhesions. If there is no, or minimal, pleural effusion in the lateral decubitus position, thoracoscopy is still possible by inducing pneumothorax via blunt dissection and the insertion of a Boutin needle prior to the thoracoscopy (21), and this allows medical thoracoscopy (under local anaesthetic and sedation) to be performed anyway, avoiding the need for surgical pleural biopsies (VATS) under general anaesthesia. Medical thoracoscopy can be safely performed as a day case procedure (22) avoiding unnecessary hospitalisation.
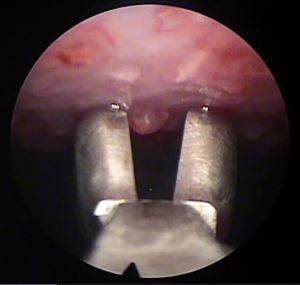
Medical thoracoscopy is safe, and well-tolerated. Major complications occur in 1.8% and include infection, haemorrhage, pneumothorax or air leak, and bronchopleural fistula, and the mortality rate is <0.5%. Other complications occur in 7.3% and include subcutaneous emphysema, on-table hypotension, and arrhythmia (20). Absolute contraindications to medical thoracoscopy include lung adherent to the chest wall throughout the hemithorax, uncontrollable cough, hypercarbia or severe respiratory distress, and the procedure should be delayed until 4 weeks after a myocardial infarction. Although avoidance of general anaesthesia is a major advantage of medical thoracoscopy, thoracoscopy performed under general anaesthesia allows more complex interventions, selective intubation and lung isolation. General anaesthesia also allows thoracoscopic visualisation of the collapsed lung being re-inflated by positive pressure ventilation allowing identification of trapped lung, thereby identifying patients who may need an indwelling pleural catheter (IPC) insertion during the same procedure (23).
Most published data are on the use of rigid thoracoscopy, however semi-rigid thoracoscopy is increasingly being used by interventional pulmonologists, providing increased flexibility and compatible with existing equipment of some flexible bronchoscopes. No significant difference has been identified between the diagnostic yields of rigid and semi-rigid thoracoscopies (24), although there are concerns about semi-rigid thoracoscopy’s ability to divide septations, and obtain biopsies in those patients with diffuse pleural thickening which might be difficult to biopsy with the semi-rigid biopsy forceps.
Other
There are isolated reports of mesothelioma diagnosis obtained by endobronchial ultrasound-guided transbronchial needle aspiration (25-28). This modality will potentially be helpful in the future if mediastinal staging becomes an important factor in deciding treatment. However, this is not the case currently, and where possible, pleural biopsy remains the diagnostic procedure of choice for mesothelioma.
Thoracoscopic pleural brushing has been used in some centres (29,30), allowing cellular samples to be obtained from areas which are considered to be high risk for biopsies, however the diagnostic yield is not as high as for pleural biopsies obtained at thoracoscopy.
Therapeutic
There is no curative treatment for mesothelioma. Chemotherapy has been shown to improve survival (31), however it is not curative. Pleurectomy was suggested to have potential benefits in some studies, although in the only randomised trial in this field, video-assisted thoracoscopic partial pleurectomy did not improve survival over talc pleurodesis in mesothelioma, and was associated with more complications, higher cost, and longer hospital stay than talc pleurodesis (32). Until evidence of clear benefits for surgery emerges, surgery is mostly recommended to be considered in mesothelioma only as part of research trials (2,3,33).
Debulking pleurectomy/decortication can relieve trapped lung by removing the visceral tumour cortex and improve symptoms such as shortness of breath and chest wall pain, however there is a lack of robust evidence to support this, and it is not recommended to be offered with curative intent. It may be considered in selected patients for symptom control especially if lung entrapment is present and therefore chemical pleurodesis contraindicated (2).
Therefore, preserving quality of life remains the main focus of care in mesothelioma patients, and interventional pulmonology has an important role in prevention of recurrence of pleural effusions and symptom control.
Therapeutic pleural aspiration
Large volume pleural fluid aspiration to relieve symptoms can be performed via bespoke pleural catheters. An analysis of patient reported outcome measures showed that 85.7% of patients with pleural effusions experience symptomatic benefit from fluid drainage, with a mean visual analogue scale (VAS) improvement of 42.6 mm, and the volume of pleural fluid drained correlates with the symptomatic benefit (34). Ultrasound enables an assessment of the hemi-diaphragm shape (flattening, inversion), position, and movement with respiration, and this can be used as a guide to the amount of fluid needing to be drained to improve symptoms (35,36). Development of persistent cough or chest pain during the procedure should be an indication to stop drainage as it may indicate development of re-expansion pulmonary oedema or underlying trapped lung. Repeated large volume pleural fluid aspiration is the treatment of choice in frail patients with <1 month life expectancy, but early definitive management of malignant pleural effusion by pleurodesis is recommended in other patients with recurrent symptomatic malignant pleural effusions (2,37), because of the high risk of fluid recurrence, the risk of adhesion formation between the visceral and parietal pleura with repeat pleural aspiration, and the potential risk of development of trapped lung in non-pleurodesis patients.
Chemical pleurodesis
Sterile talc is the most widely used and accepted sclerosant agent for chemical pleurodesis, and graded talc is rarely associated with severe complications. Its use is supported by results of meta-analyses and recommended in guidelines (37). It is useful to prevent recurrent pleural effusions although chemical pleurodesis should not be performed before obtaining sufficient tissue for diagnosis of mesothelioma (2,37). The aim of chemical pleurodesis is to obliterate the pleural space by adhesion and fibrosis of the plural layers. It has a reported 75% success rate (38). Successful talc pleurodesis is defined as no fluid reaccumulation till death, and partial response is reaccumulation of pleural fluid radiographically but not requiring further pleural intervention to relieve symptoms. Even in the presence of non-expandable lung, if >50% pleural apposition can be achieved then chemical pleurodesis may still be beneficial and provide symptom relief (37).
Talc can be delivered as a slurry through a chest drain after controlled fluid drainage and confirmation of no, or minimal, residual pleural fluid on chest X-ray, or as poudrage during thoracoscopy. Thoracoscopy permits a combined diagnostic and therapeutic procedure, with complete drainage of pleural fluid with low risk of complications including re-expansion pulmonary oedema, and immediate pleurodesis through on-table talc poudrage pleurodesis if the visualised pleura is diagnostic of malignancy. It is unclear whether talc poudrage at thoracoscopy is associated with improved outcomes when compared with standard talc slurry pleurodesis through a chest drain (39,40), and a trial is currently underway with the aim to answer this (41). IPC insertion during the same procedure in case of identified non-expandable lung, could facilitate early discharge of the patient from hospital and improve long-term fluid control. Even in the absence of non-expandable lung, IPC insertion after talc poudrage pleurodesis at thoracoscopy was found to be associated with shortened length of hospital stay, shortened time to pleurodesis, and avoids further pleural procedures should talc pleurodesis fail (42).
In malignant pleural effusions, there is some doubt as to the optimal chest drain size to be used in talc pleurodesis, and the results of a randomised controlled clinical trial [the 1st therapeutic interventions in malignant pleural effusion (TIME1)] showed that 12-F chest drains were associated with modest reduction in pain but were associated with higher pleurodesis failure (30% vs. 24%) when compared with 24-F chest drains (43).
Side effects of talc pleurodesis include pain in 7%, low-grade fever, and infection, although empyema is rare. Previously acute respiratory distress syndrome (ARDS) was a recognised complication of talc pleurodesis, however there were no cases of ARDS with larger particle talc in a large multicentre study (44).
IPCs
IPCs are becoming a first line alternative to chest drain insertion and talc pleurodesis. A randomised controlled trial analysing the effect of IPCs on dyspnoea in patients with malignant pleural effusions, when compared to chest drains and talc pleurodesis (TIME2 trial), showed no difference in patient-reported dyspnoea between chest drain insertion with talc pleurodesis and IPC (45). Because IPCs can be inserted as a day case procedure, this option is favoured by patients wishing to avoid hospitalisation. IPCs are also an important option in patients who have previous failed talc pleurodesis, and in patients with trapped lung in whom talc pleurodesis is unlikely to be successful because of a lack of visceral and parietal pleura apposition.
The patients, their carer or district nurses then perform pleural fluid drainage via the IPC intermittently at the patient’s home. There is evidence that daily drainage leads to higher rates of autopleurodesis and shorter time interval till IPC removal, than with less frequent drainage (46). The IPC-Plus trial results are as yet unpublished (47), however preliminary results were presented at the ERS congress in Milan in 2017, and an increase in pleurodesis success rate at five weeks was reported when comparing IPC with placebo (about 20% pleurodesis success rate) to IPC with talc instilled through the IPC (about 40% pleurodesis success rate) as an outpatient.
Patients undergoing chemotherapy for mesothelioma can still have an IPC inserted, although delay of the procedure until recovery of chemotherapy-related neutropenia and thrombocytopenia is advisable (48,49). Complications are uncommon and include pleural infection, although this is uncommon and usually controlled with antibiotics, pain at the IPC insertion site which usually resolves within days, pneumothorax, drain blockage, and malignant seeding along the catheter (50). A patient’s social and psychological context need to be considered prior to IPC insertion to ensure that the drainage will be managed effectively.
It is important to note that much of the reported data for IPCs comes from studies of malignant pleural effusions rather than specifically mesothelioma-associated pleural effusions, and therefore results cannot necessarily be extrapolated to mesothelioma-associated effusion.
Future directions
Traditionally pleural biopsies are required for mesothelioma diagnosis, but with further extensive research in biomarkers, biopsies may one day soon no longer be a requirement for diagnosing mesothelioma.
Non-expandable lung is usually identified on a post-pleural aspiration chest X-ray by the presence of a hydropneumothorax if adequate volumes of fluid have been drained. Prediction of non-expandable lung would be useful to plan further procedures to control pleural fluid, and if detected, IPC would be the preferred option for fluid control. The routine use of pleural manometry is somewhat controversial. Some practitioners advocate its routine use during thoracocentesis because it can detect non-expandable lung and is safe (51,52), however pressure readings have not been shown to be consistently associated with development of chest discomfort or re-expansion pulmonary oedema, and equipment is traditionally complicated. However, the development of modern advanced technology such as hand-held digital manometers may improve its availability, although further studies in this area are required to assess appropriate patient selection for this type of device (53).
Conclusions
Interventional pulmonologists are performing increasingly complex pleural interventions and offering a wider range of options for diagnosis and symptom control in mesothelioma patients, and in most cases, avoid the higher risk associated with more invasive surgical procedures.
Acknowledgments
Funding: Najib M Rahman is funded by the NIHR Oxford Biomedical Research Centre.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (David Waller and Annabel Sharkey) for the series “Mesothelioma Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.04.01). The series “Mesothelioma Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- British Thoracic Society Standards of Care Committee. BTS statement on malignant mesothelioma in the UK, 2007. Thorax 2007;62:ii1-19. [PubMed]
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [PubMed]
- Baas P, Fennell D, Kerr KM, et al. Malignant pleural mesothelioma: ESMO clinical practice guidelines for diagnosis, treatment and follow up. Ann Oncol 2015;26:v31-9. [PubMed]
- Greillier L, Baas P, Welch JJ, et al. Biomarkers for malignant pleural mesothelioma: current status. Mol Diagn Ther 2008;12:375-90. [PubMed]
- Creaney J, Dick IM, Meniawy TM, et al. Comparison of fibulin-3 and mesothelin as markers in malignant mesothelioma. Thorax 2014;69:895-902. [PubMed]
- Havelock T, Teoh R, Laws D, et al. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii61-76. [PubMed]
- Qureshi NR, Rahman NM, Gleeson FV. Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax 2009;64:139-43. [PubMed]
- Rakha EA, Patil S, Abdulla K, et al. The sensitivity of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Diagn Cytopathol 2010;38:874-9. [PubMed]
- Brims FJH, Meniawy TM, Duffus I, et al. A novel clinical prediction model for prognosis in malignant pleural mesothelioma using decision tree analysis. J Thorac Oncol 2016;11:573-82. [PubMed]
- Hooper C, Lee YC, Maskell N, et al. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii4-17. [PubMed]
- Boutin C, Rey F. Thoracoscopy in pleural malignant mesothelioma: a prospective study of 188 consecutive patients. Part 1: Diagnosis. Cancer 1993;72:389-93. [PubMed]
- Canto A, Rivas J, Saumench J, et al. Points to consider when choosing a biopsy method in cases of pleurisy of unknown origin. Chest 1983;84:176-9. [PubMed]
- Adams RF, Gray W, Davies RJ, et al. Percutaneous image-guided cutting needle biopsy of the pleura in the diagnosis of malignant mesothelioma. Chest 2001;120:1798-802. [PubMed]
- Metintas M, Yildirim H, Kaya T, et al. CT Scan-Guided Abrams' Needle Pleural Biopsy versus Ultrasound-Assisted Cutting Needle Pleural Biopsy for Diagnosis in Patients with Pleural Effusion: A Randomized, Controlled Trial. Respiration 2016;91:156-63. [PubMed]
- Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet 2003;361:1326-30. [PubMed]
- Hallifax RJ, Corcoran JP, Ahmed A, et al. Physician-based ultrasound-guided biopsy for diagnosing pleural disease. Chest 2014;146:1001-6. [PubMed]
- Koegelenberg CF, Irusen EM, von Groote-Bidlingmaier F, et al. The utility of ultrasound-guided thoracentesis and pleural biopsy in undiagnosed pleural exudates. Thorax 2015;70:995-7. [PubMed]
- Rahman NM, Ali NJ, Brown G, et al. Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii54-60. [PubMed]
- Page RD, Jeffrey RR, Donnelly RJ. Thoracoscopy: a review of 121 consecutive surgical procedures. Ann Thorac Surg 1989;48:66-8. [PubMed]
- Dixon G, de Fonseka D, Maskell N. Pleural controversies: image guided biopsy vs. thoracoscopy for undiagnosed pleural effusions? J Thorac Dis 2015;7:1041-51. [PubMed]
- Corcoran JP, Psallidas I, Hallifax RJ, et al. Ultrasound-guided pneumothorax induction prior to local anaesthetic thoracoscopy. Thorax 2015;70:906-8. [PubMed]
- Psallidas I, Corcoran JP, Fallon J, et al. Provision of day-case local anaesthetic thoracoscopy. Chest 2017;151:511-2. [PubMed]
- Bhatnagar R, Corcoran JP, Maldonado F, et al. Advanced medical interventions in pleural disease. Eur Respir Rev 2016;25:199-213. [PubMed]
- Dhooria S, Singh N, Aggarwal AN, et al. A randomized trial comparing the diagnostic yield of rigid and semirigid thoracoscopy in undiagnosed pleural effusions. Respir Care 2014;59:756-64. [PubMed]
- Hamamoto J, Notsute D, Tokunaga K, et al. Diagnostic usefulness of endobronchial ultrasound-guided transbronchial needle aspiration in a case with malignant pleural mesothelioma. Intern Med 2010;49:423-6. [PubMed]
- Kang B, Kim MA, Lee BY, et al. Malignant Pleural Mesothelioma Diagnosed by Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration. Tuberc Respir Dis (Seoul) 2013;74:74-8. [PubMed]
- Lococo F, Rossi G, Agostini L, et al. “Dry” pleural mesothelioma successfully diagnosed on endobronchal ultrasound (EBUS)-guided transbronchial needle aspiration (TBNA). Intern Med 2014;53:467-9. [PubMed]
- Guinde J, Laroumagne S, Kaspi E, et al. Endobronchial ultrasound in the diagnosis of malignant pleural mesothelioma. Rev Mal Respir 2015;32:750-4. [PubMed]
- Bejui-Thivolet F, Guérin JC, Salle M, et al. Thoracoscopy with pleural brushing. A new diagnostic method for pleural diseases. Rev Pneumol Clin 1984;40:311-9. [PubMed]
- Shaaban LH, Ahmed Y. Value of thoracoscopic pleural brush in the diagnosis of exudative pleural effusion. Egypt J Chest Dis Tuberc 2012;61:385-9. </jrn>.
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [PubMed]
- Rintoul RC, Ritchie AJ, Edwards JG, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet 2014;384:1118-27. [PubMed]
- Datta A, Smith R, Fiorentino F, et al. Surgery in the treatment of malignant pleural mesothelioma: recruitment into trials should be the default position. Thorax 2014;69:194-7. [PubMed]
- Psallidas I, Yousuf A, Talwar A, et al. Assessment of patient-reported outcome measures in pleural interventions. BMJ Open Respir Res 2017;4:e000171 [PubMed]
- Estenne M, Yernault JC, De Troyer A. Mechanism of relief of dyspnea after thoracocentesis in patients with large pleural effusions. Am J Med 1983;74:813-9. [PubMed]
- Wang JS, Tseng CH. Changes in pulmonary mechanics and gas exchange after thoracentesis on patients with inversion of a hemidiaphragm secondary to large pleural effusion. Chest 1995;107:1610-4. [PubMed]
- Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii32-40. [PubMed]
- Stefani A, Natali P, Casali C, et al. Talc poudrage versus talc slurry in the treatment of malignant pleural effusion. A prospective comparative study. Eur J Cardiothorac Surg 2006;30:827-32. [PubMed]
- Dresler CM, Olak J, Herndon JE, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005;127:909-15. [PubMed]
- Xia H, Wang XJ, Zhou Q, et al. Efficacy and safety of talc pleurodesis for malignant pleural effusion: a meta-analysis. PloS One 2014;9:e87060 [PubMed]
- Bhatnagar R, Laskawiec-Szkonter M, Piotrowska HE, et al. Evaluating the efficacy of thoracoscopy and talc poudrage versus pleurodesis using talc slurry (TAPPS trial): protocol of an open-label randomised controlled trial. BMJ Open 2014;4:e007045 [PubMed]
- Boujaoude Z, Bartter T, Abboud M, et al. Pleuroscopic pleurodesis combined with tunneled pleural catheter for management of malignant pleural effusion: a prospective observational study. J Bronchology Interv Pulmonol 2015;22:237-43. [PubMed]
- Rahman NM, Pepperell J, Rehal S, et al. Effect of Opioids vs NSAIDs and Larger vs Smaller Chest Tube Size on Pain Control and Pleurodesis Efficacy Among Patients With Malignant Pleural Effusion: The TIME1 Randomized Clinical Trial. JAMA 2015;314:2641-53. [PubMed]
- Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet 2007;369:1535-9. [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion. The TIME2 randomised controlled trial. JAMA 2012;307:2383-9. [PubMed]
- Wahidi MM, Reddy C, Yarmus L, et al. Randomized Trial of Pleural Fluid Drainage Frequency in Patients with Malignant Pleural Effusions. The ASAP Trial. Am J Respir Crit Care Med 2017;195:1050-7. [PubMed]
- Bhatnagar R, Kahan BC, Morley AJ, et al. The efficacy of indwelling pleural catheter placement versus placement plus talc sclerosant in patients with malignant pleural effusions managed exclusively as outpatients (IPC-PLUS): study protocol for a randomised controlled trial. Trials 2015;16:48. [PubMed]
- Hak CC, Sivakumar P, Ahmed L. Safety of indwelling pleural catheter use in patients undergoing chemotherapy: a five-year retrospective evaluation. BMC Pulm Med 2016;16:41. [PubMed]
- Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax 2011;66:448. [PubMed]
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011;26:70-6. [PubMed]
- Feller-Kopman D. Point: should pleural manometry be performed routinely during thoracentesis? Yes. Chest 2012;141:844-5. [PubMed]
- Folch E, Mahajan A, Majid A. Pleural manometry: ready for prime time. J Bronchology Interv Pulmonol 2013;20:297-8. [PubMed]
- Lee HJ, Yarmus L, Kidd D, et al. Comparison of pleural pressure measuring instruments. Chest 2014;146:1007-12. [PubMed]
Cite this article as: Asciak R, Rahman NM. Modern diagnostic and therapeutic interventional pulmonology in mesothelioma. Shanghai Chest 2018;2:28.