
Is more better?—for mediastinal lymph nodes this issue is still not dissected
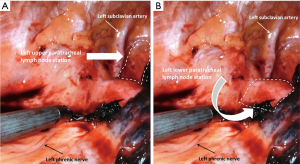
In this issue of Shanghai Chest, Kostoulas and Papagiannopoulos discuss “Mediastinal lymph node dissection in open thoracic surgery” giving a useful and concise review during thoracotomy approach for non-small cell lung cancer (NSCLC) resection. The article focuses on practical tips and pitfalls to avoid during lymph node harvest. Referencing the European Society of Thoracic Surgery guidelines on intraoperative lymph node staging and a review article by Whitson et al. which refers to the National Comprehensive Cancer Network guidelines of mediastinal lymph node evaluation (1,2), the authors state that “the gold standard for lung cancer treatment is lung resection combined with complete lymph node dissection of at least three mediastinal (N2) stations”. The article describes the anatomical landmarks and borders of each mediastinal nodal station with exception of the left upper and lower paratracheal regions. Dissection of the lower left paratracheal station requires division of the ligamentum arteriosum to allow elevation and dissection under the aortic arch. The left upper paratracheal station is bordered by the chest apex superiorly, the upper manubrium medially and the upper edge of the aorta inferiorly (see Figure 1A). The left lower paratracheal station is bordered by the trachea medially, the ligamentum arteriosum laterally, the upper edge of the aorta superiorly and the upper rim of the pulmonary artery inferiorly (see Figure 1B). Both the left phrenic and left recurrent laryngeal nerves need to be protected during this infrequent dissection. For complete margin descriptions of each lymph node station, please refer to Table 2 in the “Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer” (3).
Although complete mediastinal lymph node dissection (MLND) is described by this article, the review briefly mentions two long debated and related controversies regarding mediastinal lymph node staging for NSCLC: (I) mediastinal lymph node sampling (MLS) versus MLND and (II) lobe directed systematic lymph node dissection (L-SND) versus systematic nodal dissection (SND) stimulating further discourse. Selected lymph node biopsy, lymph node sampling, SND, lobe-specific SND, and extended lymph node dissection are defined by consensus in the ESTS intraoperative guidelines (1) (see Table 1).
Table 1
| Extent | Description |
|---|---|
| Selected lymph node biopsy | One or multiple suspicious lymph nodes are biopsied |
| Sampling | Removal of one or more lymph nodes guided by preoperative or intraoperative findings which are thought to be representative. Predetermined selection of the lymph node stations specified by the surgeon |
| Systematic nodal dissection | All the mediastinal tissue containing the lymph nodes is dissected and removed systematically within the anatomical landmarks. It is recommended that at least mediastinal nodal stations (but always subcarinal) should be excised as a minimum requirement. The hilar and intrapulmonary lymph nodes are dissected as well |
| Lobe specific systematic nodal dissection | Mediastinal tissue containing specific lymph node stations are excised, depending on the lobar location of the primary tumor |
| Extended lymph node dissection | Bilateral mediastinal and cervical lymph node dissection is performed through median sternotomy and cervicotomy |
The two studies referenced by Kostoulas and Papagiannopoulos regarding comparison of MLND versus MLS were published a decade apart from each other and therefore utilize data from different time periods. Doddoli et al. performed a retrospective review of patients at their institution from 1992–2003 and accepted a less stringent definition of MLND requiring only two mediastinal stations to be sampled but a total of at least 10 lymph nodes examined. Additionally, they included a mix of lung resections including more complex sleeve lobectomy and bilobectomy (4). Dong and colleagues performed a literature review with meta-analysis using data from 2002-2013. They included data only from pathological stage I NSCLC, eliminating the possibility of occult N1 or N2 disease. MLS in this study designated that only abnormal appearing lymph nodes were sampled whereas systematic mediastinal lymphadenectomy included removal of all lymph nodes from stations 1–4 and 7–9 on the right and 5–9 on the left (5). Regardless of the possible differences in data due to time period evaluated, both studies found improved survival with MLND compared with MLS although the previously mentioned variations in the Doddoli study may render the results less compelling. A literature review by Hughes and colleagues of studies comparing MLND and MLS found increased survival and decreased recurrence when MLND was performed for stages II and III, but not for stage I NSCLC (6). They included meta-analyses, randomized trials and cohort studies. Another meta-analysis of only randomized controlled trials of MLND versus MLS for early stage NSCLC performed by Huang and colleagues also found no difference in overall survival (OS), local recurrence, and distant recurrence or complication rates (7). Thus there are conflicting views on whether MLND affords survival advantage for early stage NSCLC and therefore no clear evidence on which to base the decision of MLND or MLS for early stage cancer.
Another controversy brought up by Kostoulas and Papagiannopoulos is whether L-SND versus SND should be performed. Except for the right middle lobe, the lymphatic drainage patterns for each lobe are well established. Lobe specific nodal dissection for a right upper lobe includes removal of the upper and lower right paratracheal lymph nodes and right hilar lymph nodes. A left upper lobe resection with L-SND means removal of the left lower paratracheal, anterior aortic, aortopulmonary window and hilar lymph nodes. Because upper lobes do not commonly drain to the subcarinal, paraesophageal or inferior pulmonary ligament nodes, these are not included for upper lobe L-SND. Conversely, for either lower lobe, the subcarinal, paraesophageal, inferior pulmonary ligament and hilar lymph nodes are included in L-SND while upper and lower paratracheal lymph nodes are not included (1,8). Adachi et al. performed a retrospective review and propensity matching of their patients who had SND and L-SND for NSCLC stage T1a-2bN0-1 resulting in 49 patients in each group. There was no difference in OS or DFS between the SND and L-SND propensity matched groups (9). Shapiro and colleagues performed a retrospective review of patients undergoing lobectomy by thoracoscopy or thoracotomy. Similar to Adachi’s findings, there was no difference in OS, disease free survival, recurrence, or morbidity between SND and L-SND in patients with N0-1 NSCLC (10). Clinical stages I and II cancers were resected by either thoracoscopy or thoracotomy in the retrospective study using the Japanese Joint Committee on Lung Cancer Registry. SND was performed in 4,124 (76.5%) patients while L-SND was performed in 1,268 (23.5%). The OS for patients with pathologic N0 (pN0) disease was better when L-SND was performed. For pN1 and pN2 patients there was no difference in OS between L-SND and SND (11). Maniwa performed a retrospective review of patients and divided them into patients who had SND (group A), patients who were selected to have L-SND (group B) due to “advanced age, severe diabetes, respiratory dysfunction and cardiovascular disease”, and patients who were selected to have L-SND (group C) with ground glass opacity, SUV on PET scan <2.5, or no elevation of CEA. Patients who had L-SND had shorter operative time. However, there was no difference in complication rates between patients having SND and L-SND. Lymph node recurrence was higher in group B than group A but this did not affect survival as 5-year OS was 89.7% and 86.6% and DFS was 77.7% and 76.4% for groups A and B respectively. Five-year OS and DFS for group C were 100% and 100% (12). There are no randomized controlled studies comparing SND and L-SND but the Lung Cancer Surgical Study Group of the Japan Clinical Oncology Group has outlined their plans for a multicenter study (13). Because of the unique precision, care and completeness of Japanese technique with lymph node harvest, the results of this upcoming trial may not be able to be generalized to other parts of the world.
In summary, Kostoulas and Papagiannopoulos have provided an excellent narrative for MLND during thoracotomy and lung resection for non-small cell lung carcinoma. The optimal oncologic extent of MLND remains an unresolved question. Further studies are needed to determine whether extent of dissection needs to be tailored to the stage and/or location of the tumor.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Dr. Jianfei Shen, MD (Department of Cardiothoracic Surgery, Taizhou Hospital of Zhejiang Province, Wenzhou Medical University, Taizhou, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.04.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Whitson BA, Groth SS, Maddaus MA. Surgical assessment and intraoperative management of mediastinal lymph nodes in non-small cell lung cancer. Ann Thorac Surg 2007;84:1059-65. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Doddoli C, Aragon A, Barlesi F, et al. Does the extent of lymph node dissection influence outcome in patients with stage I non-small-cell lung cancer? Eur J Cardiothorac Surg 2005;27:680-5. [Crossref] [PubMed]
- Dong S, Du J, Li W, et al. Systematic mediastinal lymphadenectomy or mediastinal lymph node sampling in patients with pathological stage I NSCLC: a meta-analysis. World J Surg 2015;39:410-6. [Crossref] [PubMed]
- Hughes MJ, Chowdhry MF, Woolley SM, et al. In patients undergoing lung resection for non-small cell lung cancer, is lymph node dissection or sampling superior? Interact Cardiovasc Thorac Surg 2011;13:311-5. [Crossref] [PubMed]
- Huang X, Wang J, Chen Q, et al. Mediastinal lymph node dissection versus mediastinal lymph node sampling for early stage non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2014;9:e109979 [Crossref] [PubMed]
- Han H, Chen H. Selective lymph node dissection in early-stage non-small cell lung cancer. J Thorac Dis 2017;9:2102-7. [Crossref] [PubMed]
- Adachi H, Sakamaki K, Nishii T, et al. Lobe-Specific Lymph Node Dissection as a Standard Procedure in Surgery for Non-Small Cell Lung Cancer: A Propensity Score Matching Study. J Thorac Oncol 2017;12:85-93. [Crossref] [PubMed]
- Shapiro M, Kadakia S, Lim J, et al. Lobe-specific mediastinal nodal dissection is sufficient during lobectomy by video-assisted thoracic surgery or thoracotomy for early-stage lung cancer. Chest 2013;144:1615-21. [Crossref] [PubMed]
- Hishida T, Miyaoka E, Yokoi K, et al. Lobe-Specific Nodal Dissection for Clinical Stage I and II NSCLC: Japanese Multi-Institutional Retrospective Study Using a Propensity Score Analysis. J Thorac Oncol 2016;11:1529-37. [Crossref] [PubMed]
- Maniwa T, Okumura T, Isaka M, et al. Recurrence of mediastinal node cancer after lobe-specific systematic nodal dissection for non-small-cell lung cancer. Eur J Cardiothorac Surg 2013;44:e59-64. [Crossref] [PubMed]
- Hishida T, Saji H, Watanabe SI, et al. A randomized Phase III trial of lobe-specific vs. systematic nodal dissection for clinical Stage I-II non-small cell lung cancer (JCOG1413). Jpn J Clin Oncol 2018;48:190-4. [Crossref] [PubMed]
Cite this article as: Dexter EU, Yendamuri S. Is more better?—for mediastinal lymph nodes this issue is still not dissected. Shanghai Chest 2018;2:30.


