New innovations in minimally invasive surgery—video-assisted thoracic surgery innovations from uniportal to “robotic video-assisted thoracic surgery”: technical and future aspects
Introduction
Minimal-invasive thoracic surgery mainly refers to a surgical technique involving the significant reduction of the wound access-related size. Mainly, scope surgery is the chief representative. The development of thoracoscope technique was initiated exactly one century ago by Jacobaeus (1). Diagnostic applications, such as pleural biopsy, was performed mainly by pulmonologists and, after 1990, blooming using of video-assisted surgical scope intervention all over the world led to more extensive applications to various kinds of thoracic disease. Since then, it has undergone a series of step by step modifications and improvement. Baseball diamond pattern [three-port VATS (video-assisted thoracic surgery)] introduced by Dr. Roviaro was first used to suit operational needs (2), and gradually developed into two-port VATS techniques proposed by D’amico (3). Single port video-assisted thoracic surgery (SPVATS) has a history of more than 15 years (4). In recent, SPVATS has become an increasingly popular approach to mediastinal tumor resection and major pulmonary resection (5). Robotic-assisted thoracoscopic surgery (RATS) is another representative of minimally invasive surgery, which may help overcome difficulties associated with VATS by bringing high-definition 3-dimensional (3D) vision, movements with 7 degrees of freedom and physiological tremor filtration (6).
SPVATS techniques
The experience we acquired with the SPVATS technique during the past years had gradually adopted as a standardized operation procedure:
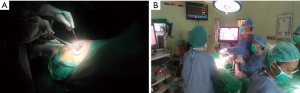
- Incision site: pivot of anterior axillary line and 5th intercostal space;
- Equipments: 30-degree endoscope and long cured instruments;
- Monitor and assistant position: video monitor is placed opposite to the surgeon and assistant stands with surgeon at the same side (Figure 1).
The main advances of SPVATS during the past years are related to improvements in surgical technique and implementation of new technology (curved tip staplers, energy devices, 3D and ultra-high definition view). Advances in visualization with the use of ultra-high definition monitors provided surgeons better operation view and vivid field of depth. Placing a 30-degree endoscope at the top side of wound give surgeons an overlook view of operation field, which is similar to open surgery. Long curved instruments were needed in single port surgery because it could reduce the possibility of instruments collision.
Image guided SPVATS
The National Lung Cancer Screening Trial has demonstrated that screening of high-risk populations with the use of low-dose computed tomography (LDCT) reduces lung cancer mortality. Based on the inspiring results, more and more small pulmonary nodules (SPNs) and ground glass opacity (GGO) lesions were found during health examination or low dose CT screening program. Small pulmonary lesions <1 cm or those at a distance from the lung periphery, especially when deeper than 2.5 times the lesion diameter, can be difficult to locate by palpation intraoperatively, let alone GGO lesions. In recent two decades, preoperative CT localization was widely used in various kinds of occult lesions, including SPNs. However, dislodgment of the hookwire from its perinodular location was the major drawback. Pneumothorax, hemothorax, and air embolism were also common complications after preoperative CT localization. Ng et al. first published their experience about one stage operation of GGO lesion in hybrid operation room (7). In recent, Hsieh et al. also described their experience of single-stage localization and removal of small lung nodules through image-guided video-assisted thoracoscopic surgery (8). Combination of hybrid operation room and thoracic endoscope surgery might open doors to new techniques that can be potentially safer, more effective, and more economical for patients. Furthermore, fluorescence image-guided technique is a highly sensitive and specific imaging method that has the potential to identify imperceptible lesions intraoperatively. Mao et al. published their experience about indocyanine green (ICG)-fluorescent-guided exploration during VATS (9). With the combination of image guided localization techniques, SPVATS still has a lot of potential fields to develop its applications.
Tubeless SPVATS
Non-intubated anesthetic techniques have progressively become more effective and safe. This kind of operation overtakes many of the limits related to the invasiveness of the surgical procedures satisfying all the parts: patient, surgeon, physician, nursing staff. Mineo et al. published their experience about 1,000 various kinds of surgery under non-intubated anesthesia, which provided substantial feasible and safety evidence for such kind an application in VATS surgery (10). Moreover, the highly innovative group from The First Affiliated Hospital of Guangzhou Medical University, China, led by Jianxing He, published an interesting study recently. Under highly selection of patients: SPNs <2 cm, body mass index (BMI) of less than 25; American Society of Anesthesiologists (ASA) grade of II or less; no history of prostate or renal disease and no parenchymal air leak at the end of surgery. No endotracheal tube, Foley, and chest tube was left at the end of surgery. It proves that tubeless SPVATS was feasible in selected patients. We still look forward for its further biological impact study in the future.
Robotic surgery
At the other aspect of minimally invasive surgery, robotic surgery develops rapidly in recent years. VATS and robotic thoracic surgery have shown better perioperative outcomes and equivalent oncologic results compared with thoracotomy. According to the National Comprehensive Cancer Network (NCCN) guidelines (version 5.2017) regarding non-small cell lung cancer (NSCLC) “VATS or minimally invasive surgery (including robotic-assisted approaches) should be strongly considered for patients with no anatomic or surgical contraindications”. With the revolution of robotic surgery system: perfect 3D view, more freedom of robotic arm than endoscope instruments, precise movement, and so on, it is easier to perform thoracic surgery by new generation robotic system. In addition, it is easy to detect isocyanides green labeled target or segmentectomy plan in robotic surgery due to its well-equipped endoscope system. In addition, robotic system also has well-established visual spatial training program, which might reduce the learning time for the novice to be familiar with robotic surgical system. However, some disadvantages still exist in robotic system: high cost, the need of another consultant on the table, multiple incisions. Therefore, fusion innovation and existing advantages, SPVATS and robotic system, could be amalgamated and stretched. We truly believed single wound incision with flexible working instruments might be the future of thoracic surgery. We have to be open to the new therapies and the next robotic era. In the future of lung cancer treatment probably will be related to individual, genetic, selective molecular chemotherapy and microrobotics technology.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lorenzo Spaggiari and Domenico Galetta) for the series “Minimally Invasive Thoracic Oncological Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.03.11). The series “Minimally Invasive Thoracic Oncological Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jacobaeus HC. Endopleural operations by means of a thoracoscope. Beitr Klin Tuberk 1915;35:1-9. [Crossref]
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic Pulmonary Lobectomy for Cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Lin TS, Kuo SJ, Chou MC. Uniportal endoscopic thoracic sympathectomy for treatment of palmar and axillary hyperhidrosis: analysis of 2000 cases. Neurosurgery 2002;51:S84-7. [Crossref] [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Ashton RC Jr, Connery CP, Swistel DG, et al. Robot-assisted lobectomy. J Thorac Cardiovasc Surg 2003;126:292-3. [Crossref] [PubMed]
- Ng CSH, Man Chu C, Kwok MWT, et al. Hybrid DynaCT scan-guided localization single-port lobectomy. Chest 2015;147:e76-8. [Crossref] [PubMed]
- Hsieh MJ, Fang HY, Lin CC, et al. Single-stage localization and removal of small lung nodules through image-guided video-assisted thoracoscopic surgery. Eur J Cardiothorac Surg 2017; [Epub ahead of print]. [PubMed]
- Mao Y, Chi C, Yang F, et al. The identification of sub-centimetre nodules by near-infrared fluorescence thoracoscopic systems in pulmonary resection surgeries. Eur J Cardiothorac Surg 2017;52:1190-6. [Crossref] [PubMed]
- Mineo TC, Tamburrini A, Perroni G, et al. 1000 cases of tubeless video-assisted thoracic surgery at the Rome Tor Vergata University. Future Oncol 2016;12:13-8. [Crossref] [PubMed]
Cite this article as: Wu CF, Gonzalez-Rivas D. New innovations in minimally invasive surgery—video-assisted thoracic surgery innovations from uniportal to “robotic video-assisted thoracic surgery”: technical and future aspects. Shanghai Chest 2018;2:32.