Robotic esophagectomy
Introduction
Minimally invasive esophagectomy (MIE) continues to increase in popularity. The term “minimally invasive” can refer to performing either or both the thoracic and abdominal phases of the operation with either the laparoscope or robot. Transhiatal esophagectomy is another form of MIE that avoids chest incision. Recent studies have demonstrated that MIE has benefits with decreased blood loss, chest tube duration, length of stay, and respiratory complications versus open esophagectomy (1-4) and maybe even reduce cost (5). Melvin et al. were the first to report robotic esophagectomy in 2002 (6). Robotic technology for either/both the abdominal and thoracic phases of the operation, whether a transhiatal, Ivor Lewis, or modified McKeown approach is taken, has become increasingly common. A nomenclature paper is underway to help define what are the different ways to perform the operation and to ensure that we are comparing similar techniques fairly.
Indications
Most candidates for esophagectomy are also candidates for attempted MIE, and therefore also, candidates for robotic esophagectomy. We have not offered an open esophagectomy to a patient in over 7 and they all are done using MIE techniques including those who have had previous surgery. There are few specific contraindications for the use of robotic technology. The need to perform an en bloc resection of aorta or intrathoracic trachea or carina along with the esophagectomy, which has been safely applied to selected patients, would generally be considered a contraindication to robotic esophagectomy (7,8). Prior thoracic or abdominal surgery can make a robotic approach more challenging due to the presence of adhesions, but lysis of adhesions can be performed in order to permit its use. Comorbidities or poor functional status that would otherwise make patients suboptimal candidates for esophagectomy generally would apply to offering robotic esophagectomy also, although robotic esophagectomy may permit surgeons to offer esophagectomy to somewhat older and sicker patients by decreasing the perioperative complication rate (especially respiratory complications) (9). We have shown that cirrhosis from alcohol abuse increased the 90-day mortality (10).
Early-stage (T1a and early T1b) esophageal cancers can be managed with endoscopic mucosal resection (EMR). Generally, if a lesion is not amenable to EMR or T1b or deeper on final pathologic analysis, esophagectomy should be considered. If EMR for early-stage esophageal cancer is performed in the context of Barrett’s esophagus, radiofrequency ablation (RFA) to promote regression of Barrett’s should be considered also. Patients with persistent high-grade dysplasia following attempted RFA are also candidates for esophagectomy. Benign indications for esophagectomy include end-stage achalasia or mega-esophagus, refractory stricture, intractable reflux resistant to surgical interventions, and multiple failed hiatal hernia operations.
Equipment
The da Vinci Surgical System is currently the only FDA-approved robotic systems for esophageal surgery. The most current Xi technology offers the surgeon the ability to autonomously staple and use infrared technology in all patients. The surgeon sits at a console some distance from the patient who is positioned on an operating table in close proximity to the robotic unit with its four robotic arms. The robotic arms incorporate remote center technology, in which a fixed point in space is defined, and about it the surgical arms move so as to minimize stress on the abdominal and thoracic wall during manipulations. The small proprietary EndoWrist instruments attached to the arms are capable of a wide range of high-precision movements. These are controlled by the surgeon’s hand movements, via “master” instruments at the console. The “master” instruments sense the surgeon’s hand movements and translate them electronically into scaled-down micro-movements to manipulate the small surgical instruments. Hand tremor is filtered out by a 6-Hz motion filter. The surgeon observes the operating field through console binoculars. The image comes from a maneuverable high-definition stereoscopic camera (endoscope) attached to one of the robot arms. The console also has foot pedals that allow the surgeon to engage and disengage different instrument arms, reposition the console “master” controls without the instruments themselves moving, and activate electric cautery. A second optional console allows tandem surgery and training. Da Vinci currently offers both the Xi and Si systems. The Xi system is newer and features an overhead beam that permits rotation of the instrument arms, allowing for greater flexibility in terms of direction of approach of the robot to the patient. Compared to the Si, the Xi also has thinner instrument arms, longer instruments themselves and the option to switch the camera to any arm/port.
Preoperative evaluation
A thorough history and physical should be performed, focusing on key points such as Barrett’s esophagus, gastroesophageal reflux disease, motility disorders such as achalasia, prior surgeries, functional status, and cardiac and respiratory comorbidities. Smoking cessation should be encouraged and alcohol use should be noted in order to screen for cirrhosis and warn of possible withdrawal issues in the perioperative period. Patients undergoing esophagectomy for neoplasm should receive whole-body PET-CT scan to evaluate for possible metastatic disease, unless this is obvious from chest/abdominal CT scans alone. Endoscopic ultrasound (EUS) plays a critical and necessary role for T status determination as well as the extent of the Barrettes and the precise location and extent of the cancer. If fairly convincing combined radiologic and clinical evidence exists for metastatic disease (e.g., weight loss, widespread adenopathy or liver/lung nodules), biopsy confirmation of metastatic disease is still usually necessary for the determination of tissue marker. Single-site M1 disease should be confirmed with tissue diagnosis. Location of tumor, synchronous lesions, and presence/extent of Barrett’s esophagus should be noted on the preoperative endoscopy. Tumors extending into the proximal stomach may require a partial gastrectomy and different reconstructive approach; tumors in the mid-esophagus should generally be approached via a McKeown type operation rather than Ivor Lewis. An adequate margin may be difficult to achieve for tumors in the proximal 1/3rd of the esophagus; these patients are better suited for definitive chemoradiation, although in some centers laryngoesophagectomy may be an option. Some investigators have suggested that patients with preoperative dysphagia may not need an EUS given that 90% of them had T3–T4 disease, a finding that has been corroborated by others (11,12).
However, although the presence of symptoms such as dysphagia is a very specific finding for the presence of a T3 or greater lesion, the absence of symptoms does not necessarily indicate that the patient does not have a T3 or greater lesion. Given that being T3 or deeper and or the presence of N1 or greater disease dictates the performance of induction chemoradiation at our institution, we also consider endoscopic ultrasound a critical part of the preoperative evaluation. Performance of induction chemoradiation for T2N0 lesions is variable. We prefer preoperative therapy since a significant percentage will have nodal disease although in the patient who is older than 75 controversy exists. Brain imaging is performed if the patient has neurological symptoms or headaches that are concerning for intracranial metastases. Bronchoscopy is done if the patient has an esophageal cancer of the proximal or middle esophagus to rule out airway invasion. Patients who remain candidates for esophagectomy after the above testing generally also receive pulmonary function testing and stress testing. No specific diagnostic procedures are performed for robotic esophagectomy per se.
After the completion of induction chemoradiation, restaging PET-CT should be performed. Patients who develop progression of disease or metastases are offered palliative management strategies. Patients who have persistent disease or show a response (complete or partial resolution of FDG avidity of the lesion on PET-CT scan) are scheduled for esophagectomy from 8–12 weeks after the conclusion of chemoradiation, once they have recovered reasonably well from the side effects of induction therapy. Data on the optimal interval between completion of chemoradiation and surgery is mixed. Kim et al. showed no difference in terms of perioperative risk, pathologic response, or overall survival between patients who were resected more than 8 weeks after chemoradiation versus those resected less than 8 weeks after (13). Lee et al. demonstrated that prolonging the interval after chemoradiation for esophageal adenocarcinoma increased the pathologic complete response rate to induction therapy; however, this did not translate to survival (14). Chiu et al., though, found that delayed surgery (defined as >8 weeks after chemoradiation) was associated with decreased 5-year survival for patients with squamous cell carcinoma that demonstrated a complete clinical response (15).
Choice of operation
The abdominal and/or thoracic phase of the esophagectomy can be done with robotic assistance. Choice of type of esophagectomy (Ivor Lewis, Mckeown, or transhiatal) can be surgeon-dependent, with some preferring a neck anastomosis due to the decreased incidence of mediastinal leaks, and others preferring a chest anastomosis due to the risk of recurrent laryngeal nerve injury. The neck clearly increases the risk of leak and the risk of recurrent laryngeal nerve injury. Location of the tumor as well as tissue type dictates this decision as well; for instance, a mid-thoracic squamous cell tumor is best suited for resection of the entire intrathoracic esophagus with a neck anastomosis.
Technical details
Abdominal phase
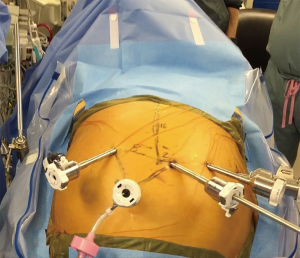
The operation starts in the abdomen for Ivor Lewis of transhiatal esophagectomies. Port placement is shown in . The camera port is located 16 cm inferior from the xiphoid process and 3 cm laterally to the right and is generally placed first. A 30 degree down camera or a 0-degree lens is used we prefer a 0. Inspection of the abdomen is performed for liver and peritoneal metastases prior to placing the other ports. A single left robotic arm and two right robotic arms are used. These ports should be placed no more than 2–3 cm superior to the camera port, in order to avoid problems with the angle of the instruments when dividing the greater omentum off the greater curvature of the stomach towards the pylorus. If the J tube is to be performed with the robot lower port placement is better. The robotic arms should be around 9 cm apart from each other if a Si system is used (8 cm if an Xi system is used) and this is measured after, after the abdomen is fully inflated with CO2. If there is not enough room on the left side of the abdomen to place the ports straight across, the robotic arm closer to the camera can be staggered slightly in front of the other one. If using the Si system, the 2nd right robotic arm can be a 5 mm and the other robotic arms 8 mm. Stapling of the conduit will need to occur via the assistant port (as shown in Figure 1). If using the Xi system and robotic stapling is desired, the left robotic arm should be a 12-mm port; the rest of the robotic ports are 8-mm ports. A 5-mm port for the liver retractor is placed as close to the costal margin and laterally as possible (just over the right colon). A 12-mm assistant port is placed in the patient’s right lower quadrant, triangulated behind the left robotic arm port and camera port. Insufflation should be delivered via this port during the case. We favor a CO2 insufflation system that autoregulates the air pressure and helps prevent smoke and camera fogging (Air Seal System, ConMed, Utica, NY, USA).
The patient is placed in steep reverse Trendelenburg position after the robot is brought over the patient’s head and before docking and the liver retractor is positioned under the left lateral lobe of the liver in order to expose the esophageal hiatus. We use a Snowden Pencer articulating pretzel retractor (Becton Dickinson; Franklin Lakes, NJ, USA) for this purpose. If using a Si system, the head of the bed is turned so that the robot can approach it from over the head. If using an Xi system, the bed does not have to be turned. The robot is carefully driven in, making sure that its arms do not collide with the patient’s head and upper body. The robotic arms are docked to the ports and the robotic phase begins. Reverse Trendelenburg is not established until after the robot is driven in over the patient’s head.
We generally use the following instruments during the dissection: left robotic arm—Cadiere forceps, right robotic arm—bipolar specially the long bipolar grasper or vessel sealer, second right robotic arm—thoracic grasper (Si system), tip-up fenestrated grasper (Xi system). Division of the greater omentum from the greater curvature stomach starts by entering the lesser sac between the stomach and the left side of the transverse colon. Care is taken to identify the gastroepiploic vessels and assiduously avoid them. The greater omentum is divided from the patient’s left towards the right until the pylorus is reached. The surgeon then switches direction and goes from the entry point into the lesser sac towards the spleen and fundus, paying extra attention to the short gastric vessels. During this process, the 2nd robotic arm is used to hold the greater omentum/colon in one direction; the assistant can grasp the stomach and retract in the other direction. An omental flap should be preserved during this dissection to be wrapped around the anastomosis and protect the airway. Once the short gastric vessels are divided, the surgeon then works on the left side of the esophageal hiatus, working from the top of the hiatus down underneath the esophagus so that the area beneath the esophagus is clear as possible in order to facilitate encircling the esophagogastric junction later. Attachments of the stomach to the retroperitoneum should also be divided at this point.
Next, the lesser sac is entered through the lesser omentum. An accessory or replaced left hepatic artery originating from the left gastric artery can be located in this area, as up to 12% of patients may have this variation (16). We then perform a circumferential dissection around the esophagus at the hiatus, carrying it into the mediastinum for a few centimeters but trying to avoid excessive trauma or widening of the hiatus or entering the left pleura, to avoid increasing the risk of paraconduit herniation. If a transhiatal esophagectomy is planned, however, this mediastinal dissection should be performed as high as possible. The use of the fenestrated bipolar forceps in the right hand can be helpful for atraumatically dissecting the underside of the esophagus. During this, the 2nd right robotic arm is used to retract the esophagus up and to the patient’s left. A 1” thick or greater Penrose drain is placed around the esophagus and the ends are either tied or stapled together. The left gastric pedicle is identified and the surrounding fat is dissected off the vein and artery. Depending on the patient, this vessel can be approached either from the lesser curvature side, or from underneath the stomach as it is lifted up (greater curvature side). Test clamp of the pedicle is performed.
Next, botulinum toxin injection with 100 units in 4 mL of saline at the pylorus is performed. Alternatively, a gastric emptying procedure such as pyloromyotomy or pyloroplasty may be done at the discretion of the surgeon, although this increases operative time without a significant improvement in results (17) and risks life-long bile reflux. The pylorus should be able to reach the esophageal hiatus with little tension; if tension exists or it cannot reach, further mobilization of the pylorus from the greater omentum and a Kocher maneuver should be performed. Care should be taken to avoid injuring structures in the portal triad during mobilization. At this point, the surgeon needs to confirm that the nasogastric tube has been withdrawn to 20 cm or so. A starting point on the lesser curvature of the stomach is selected, and the perigastric fat going from the edge of the stomach to the opening in the lesser omentum is divided with the vessel sealer. The gastric conduit is created with a stapler (4 mm staple height, 45–60 mm length), using right robotic arm #2 (placed on the fundus) and the assistant (grasping or retracting the antrum) to stretch the stomach out. The specimen is not completely divided from the conduit, so that the conduit may be pulled up into the chest or neck with the abdomen. A suture is placed at the distal part of the staple line, near the pylorus, so that the end of the staple line can be easily seen from the chest. If a neck anastomosis is being performed, part of the lesser curve of the specimen may be resected at this point in order to help facilitate its passage through the thoracic inlet. The Penrose drain and specimen are pushed up into the mediastinum.
Finally, a jejunostomy tube is placed in selective patients. We have been moving more towards a selective approach for our J tube as they add significant morbidity and emergency room visits that lead to low patient satisfaction. If the anatomy is suitable (e.g., exposure to the proximal jejunum is in front of the camera rather than directly below or behind it), this can be done robotically. Generally, though, we have found it simpler and quicker to perform this laparoscopically after undocking the robot. Three 2-0 absorbable sutures are placed in the proximal jejunum in a triangulated fashion and the ends are exteriorized. The jejunostomy tube is placed with a Seldinger technique over a wire after dilating the tract and the sutures are tied.
Thoracic phase
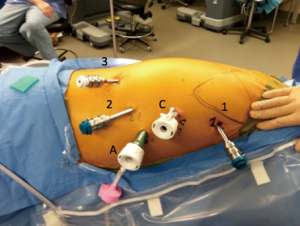
The single lumen endotracheal tube may be exchanged for a double lumen endotracheal tube during closure of the skin incisions to expedite transition to the thoracic phase. The patient is positioned in a nearly prone position. It is important to keep the right arm/shoulder close to the left arm and elevated during positioning so it will not get in the way of the right robotic arm. Patient positioning and port placement in demonstrated in Figure 2. The right robotic arm port (8 mm; 12 mm if completely robotic linear stapling technique is desired) is placed in the axilla. A long trocar can be used to help avoid collisions with the patient. The camera port is placed about 9 cm (10 cm in Si) from the right robotic arm port (8 mm in Xi system, 12 mm in Si system), in line with the anterior superior iliac spine. The left robotic arm port (12 mm in Xi if completely robotic linear stapling of anastomosis is desired, 8 mm if not desired or in Si system) is placed 9 cm (10 cm in Si) away from the camera port in line with the anterior iliac spine. The 2nd left robotic arm port (8 mm in Xi, 5 mm in Si) is placed posterior to the mid-axillary line just above the diaphragm. The assistant port (12 mm) is placed in a position triangulated behind the left robotic arm and camera port just above the diaphragm. We generally use the following instruments during the dissection: left robotic arm—Cadiere forceps, right robotic arm—thoracic dissector, second right robotic arm—thoracic grasper (Si system), tip-up fenestrated grasper (Xi system). The lung is retracted anteriorly with the 2nd left robotic arm and the inferior pulmonary ligament is divided. Lymph nodes from stations 8 and 9 are resected. The esophagus is dissected off the pericardium, until the carina is exposed. Lymph nodes from station 7 are resected so that the right mainstem bronchus, carina, and left main stem are clearly visible. Thermal injury to the airway is carefully avoided. The dissection is carried towards the azygos vein, which is then isolated and divided with a vascular load staple fire as posteriorly as possible. The dissection of the esophagus is then carried proximally and into the thoracic inlet, now staying closer to the esophagus to avoid a tracheal injury. If performing a cervical anastomosis, this dissection is carried as high as possible. Next, the esophagus is dissected off the aorta posteriorly, taking care to avoid the thoracic duct, which runs especially close to the esophagus near the azygos vein. This is carried from the thoracic inlet towards the diaphragm until the dissection plane from the abdominal phase is reached and the Penrose drain is grasped. The Penrose drain can then be retracted with the 2nd left robotic both anteriorly and laterally in order to help facilitate the remainder of the dissection. We try not to enter the left pleural space unless this needs to be resected for gross disease.
If a Mckeown esophagectomy the chest phase is performed first. The Penrose is pushed up into the thoracic inlet to be retrieved through the neck. Much of the distal neck esophagus can be dissected in the chest with a right robotic approach we believe that this is an added advantage of a robotic approach and may significantly decrease the incidence of recurrent laryngeal nerve injury. The chest tube is placed (additional tube placed in left pleural space if entered), insufflation gas turned off, ports removed, port sites are checked for bleeding, the lung reinflated, and incisions closed. Then the abdominal and cervical phases of the operation are begun.
If an Ivor Lewis esophagectomy is to be performed, the specimen and conduit is brought up into the thorax, mostly with the assistant using an atraumatic grasper such as empty ring forceps, making sure that the conduit is not twisted (staple line directed laterally). The previously placed suture at the distal staple line in the lesser curve should be visible and brought just into the chest past the hiatus. This is critical as it ensures the stomach is straight and taunt and not redundant. The esophagus is transected at the desired length with robotic shears and proximal and distal margin is sent for pathologic examination. The specimen is divided from the gastric conduit with the stapler (4 mm staple height), and together with the Penrose removed. The gastric conduit is oriented so that the anastomosis will be located posteriorly. The conduit is tacked to pleura and/or transected vagus nerve to keep it in placed during the anastomosis and to orient it and to prevent tension. The anastomosis can be completely hand-sewn, completely stapled (linear or circular stapler), or a combination of the two (linear stapler “posterior” wall and hand-sewn “anterior” wall). The optimal approach to performing the anastomosis has not been described. We have performed all of these and will describe each technique but we now prefer after over 150 consecutive robotic esophagectomies a linear stapled posterior anastomosis with a hand sewn anterior part using a running barbed locking suture. This has eliminated strictures in our practice and has a leak rate of 1.4%.
- Hand-sewn anastomosis;
- The gastrotomy is made on the posterior wall of the conduit for an “end-to-side” anastomosis, at least 2 cm proximal to the tip of the conduit and away from the staple line;
- A row of 3-0 silk sutures is placed for the outer layer (“posterior”);
- An inner layer of 3-0 absorbable sutures is placed for the inner layer (“posterior”);
- The “anterior” wall for the anastomosis is closed with interrupted 3-0 absorbable sutures;
- An outer “anterior” layer of 3-0 silk sutures is placed.
- Combination stapled and hand-sewn anastomosis;
- After the gastrotomy is performed, the esophagus and conduit are lined up and a stapler is fired to create a 20–30 mm common wall for the “posterior” wall for the anastomosis. The stapler can be deployed either by the assistant or in the left robotic arm (Xi system);
- The “anterior” wall is closed in two layers as described above.
- Completely stapled anastomosis (linear stapler);
- The “posterior” wall of the anastomosis is created as described above;
- Five 3-0 silk interrupted sutures are placed and tied to approximate the muscle and mucosa of the “anterior” wall;
- These sutures are held up and the “anterior” wall of the anastomosis is created with the linear stapler. The stapler generally needs to be deployed in the location of the right robotic arm port (either robotically, in which case the surgeon needs to upsize to a 12-mm port, or after undocking that port and having the assistant place a stapler through it).
- Completely stapled anastomosis (circular stapler);
- A purse string with 3-0 non-absorbable monofilament sutures is placed in the esophagus, making sure to incorporate the mucosal layer;
- The anvil is placed in the esophagus and the purse string tied;
- An additional purse string suture is placed if there is any gap around the anvil;
- A gastrotomy is created at the tip of the conduit. Retraction sutures can be placed to help;
- The stapler is positioned through the gastrotomy with the end directed towards the posterior aspect of the conduit. The tip of the stapler is extended, going through the wall of the conduit. Caution should be exercised to avoid deployment of the tip of the stapler into the aorta. Multiple attempts at extending the tip through the conduit wall should also be avoided. The tip of the stapler is linked to the anvil, which can be facilitated with the use of the laparoscopic anvil grasper;
- The stapler is fired. The rims of tissue excised by the stapler should be examined; if they are not complete, the corresponding area of the anastomosis should be checked and closed with sutures.
The anastomosis should be inspected and any questionable areas should have sutures placed to close them. Endoscopy can be performed routinely if so desired and the integrity of the anastomosis checked by insufflating it while it is submerged. The omental flap is wrapped around the anastomosis, protecting the airway from it, and sutured in place. The gastric conduit is secured to the diaphragm at the hiatus. A chest tube is placed and this phase of the operation concluded as described before.
Cervical phase
The cervical phase of the operation is similar to that performed during open operations. During the thoracic phase of a Mckeown esophagectomy, we perform extensive periesophageal dissection into the thoracic inlet. We find it helpful to place a Penrose drain around the esophagus and either staple or tie the ends together, and push the drain into the superior mediastinum, so that the esophagus can be more easily encircled during the neck dissection. An incision anterior and parallel to the left sternocleidomastoid is made. The platysma is divided. The omohyoid muscle is encountered and divided. The carotid sheath containing the common carotid artery, internal jugular vein is gently retracted laterally. The trachea is gently retracted medially, taking care to avoid the use of metal retractors that could injure the recurrent laryngeal nerve in the tracheoesophageal groove. The esophagus is followed inferiorly until the intrathoracic dissection plane is reached and the Penrose drain is identified. The Penrose is used to the pull the esophagus into the incision. The specimen and conduit are pulled gently up through the chest. This can be done with the camera in the abdomen to make sure that the conduit does not twist during the process, and to facilitate passage of the lesser curvature of the specimen or omental fat attached to conduit through the hiatus. The esophagus is then divided sharply, and the specimen is stapled off the gastric conduit, taking care to prevent the conduit from retracting back into the mediastinum with a non-traumatic clamp. A gastrotomy is made in the conduit on the posterior wall and a 3.5–4.5 mm tall stapler is fired to create the posterior wall of the anastomosis. Interrupted silk sutures are placed and tied anteriorly to approximate the mucosa and muscle, and a stapler is used to create the anterior wall of the anastomosis, making sure to incorporate mucosa of both the esophagus and stomach along the entire edge. If there is concern about the integrity of the anastomosis and enough redundancy exists, a buttressing layer over the staple line can be created by Lembert type interrupted 3-0 silk sutures. We do not routinely test the anastomosis for leak intraoperatively or leave a nasogastric tube. We do not typically leave a drain in place. The incision is then closed in layers with absorbable suture.
Postoperative management
During the operation a Jejunostomy tube is placed and feeding is initiated on postoperative day 1 at a rate of 10 cc/h. and increased over the next 24–48 h. As long as the patient doesn’t develop ileus or significant distention. Removing chest tube is done after verifying no chylothorax or gastric effusion and chest tube amylase level is less than 200 IU/L on postoperative day 3 (18,19).
Also, a speech evaluation is done by fiber-optic esophageal evaluation of swallowing (FEES) postoperative day 3, 4 or 5, if it’s normal a barium swallow is performed to assess gastric emptying. If all tests are normal the patients are discharged home on clear fluids or less commonly a soft diet with jejunal feeding to meet daily-recommended nutritional support at a median of 8 days postoperative robotic assisted Ivor Lewis esophagectomy (20,21).
Results
The results from series of robotic esophagectomy to date are shown in Table 1, and compared to the largest non-robotic MIE series currently in the literature (33). The largest series (from Sarkaria and Cerfolio, N=270) is scheduled to be reported in November 2017 with plans for submission into Annals of Thoracic Surgery. Overall operative times of robotic esophagectomy have been comparable to non-robotic MIE; a significant improvement in speed after the first 20 cases has been described (22). Perioperative morbidity and postoperative parameters have also been similar to that reported from series of non-robotic MIE. The single retrospective study comparing robotic esophagectomy with non-robotic MIE showed similar operative times, estimated blood loss, resected lymph nodes, postoperative length of stay, and complications (3). Direct comparisons of robotic esophagectomy with open esophagectomy have not been reported in the literature, but one randomized trial (ROBOT trial) is currently accruing patients to investigate differences in outcome between the techniques (34). The robotic platform allows for real-time assessment of perfusion of the gastric conduit with the injection of indocyanine green (ICG) and near-infrared fluorescence imaging, which can help guide the surgeon to optimal area of transection of the specimen from the conduit and also for placement of the anastomosis. Investigators have described a 0% leak rate in 39 cases after instituting routine perfusion assessment using ICG to guide creation of the esophagotomy and performance of the anterior part of the anastomosis during the thoracic phase of Ivor Lewis esophagectomy, although it is possible that improvements in anastomotic technique also contributed to this remarkable result (30). The use of ICG and near-infrared fluorescence imaging can also help with assessment of the vascular arcade during mobilization of the gastric conduit during the abdominal phase of the operation (35).
Table 1
| Author, year | #Patients | Lymph nodes dissected (median) | Operative approach | Estimated blood loss (mL) | Operative time (min) | Leak rate | Overall major morbidity | Mortality |
|---|---|---|---|---|---|---|---|---|
| Cerfolio et al., 2016 (10) | 85 | 22 | Ivor Lewis (lap/robot abd, robot chest) | 35 | 361 | 4.3% | 36.4% | 3.5% 30-day; 11% 90-day |
| Hernandez et al., 2013 (22) | 52 | 20 | Ivor Lewis (robot abd/chest) | NR | 442 | 3.8% | 26.9% | 0% (“hospital”) |
| de la Fuente et al., 2013 (23) | 50 | 18.5 | Ivor Lewis (robot abd/chest) | NR | 445 | 4% | 28% | 0% (“hospital”) |
| Sarkaria et al., 2013 (24) | 21 | 20 | Ivor Lewis (n=17) and Mckeown (n=4), robot abd/chest | 300 | 556 | 14% (grade II or greater) | 24% (grade III or greater) | 4.8% (“postoperative”) |
| Dunn et al., 2013 (25) | 40 | 20 | Transhiatal (robot mediastinal dissection) | 100 | 311 | 25% | NR | 2.5% 30-day |
| Weksler et al., 2012 (3) | 11 | 19 | Mckeown (robot abd/chest) | 200 | 445 | 9.1% | 36.4% | 0% (“hospital”) |
| Park et al., 2016 (26) | 114 | 44 | Mckeown (lap/robot abd, robot chest) | 209 | 420 | 12.3% (grade II or greater) | NR | 2.5% 90-day |
| Boone et al., 2009 (27) | 47 | 29 | Ivor Lewis (lap abd, robot chest) | 625 | 450 | 21% | NR | 6.4% (“postoperative”) |
| Kernstine et al., 2007 (28) | 14 | 18 | Ivor Lewis (lap/robot abd, robot chest) | 275 | 11.2 hours (total room time) | 14% | 29% | 0% 30-day; one patient (7.1%) died at 72 days |
| Galvani et al., 2008 (29) | 18 | 14 | Transhiatal (robot abd) | 54 | 267 | 33% | NR | 0% 30-day |
| Hodari et al., 2015 (30) | 54 | 16 | Ivor Lewis (lap abd, robot chest) | 74.4 | 362 | 6.8% | NR | 2% 30-day |
| Coker et al., 2014 (31) | 23 | 15 | Transhiatal (robot abd) | 100 | 231 | 9% | NR | 4% 30-day |
| Harrison et al., 2015 (32) | 43 | 12 | Transhiatal (n=28), Mckeown (n=7), Ivor Lewis (n=5); robot abd | NR | 309 | 23% | 41.8% | 4.7% (“postoperative”) |
| Luketich et al., 2012 (33) | 1,033 | 21 | Mckeown (n=481), Ivor Lewis (n=530) Lap abd/VATS chest | NR | NR | 5% (requiring surgery) | NR | 1.68% 30-day; 2.8% 30-day or hospital |
NR, not reported; VATS, video-assisted thoracoscopic surgery.
Some of the advantages of robotic esophagectomy over non-robotic MIE may be difficult to quantify, and relate to subjective experiences of the surgeon such as the 3-dimensional nature of the optics, improved dexterity, favorable ergonomics, and the ability to control the retraction and camera without an assistant (36,37). Disadvantages of the robotic platform include cost, and complexity in terms of developing robotic skills, personnel issues, room layout and robot docking, although these may be surmounted with a formal training paradigm (38). Long-term oncologic outcomes specific to robotic esophagectomy are not yet well described, although it would be expected that they would comparable to those for non-robotic MIE.
Conclusions
Robotic esophagectomy can be done safely with comparable intraoperative parameters, morbidity, and outcomes to non-robotic MIE, while offering certain more subjective advantages to the surgeon. Further studies are needed to show the true benefits of MIE but if equivalent, as it seems to be at least, then most educated patients and surgeons will continue to seek and learn how to perform robotic MIE.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lorenzo Spaggiari and Domenico Galetta) for the series “Minimally Invasive Thoracic Oncological Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: The series “Minimally Invasive Thoracic Oncological Surgery” was commissioned by the editorial office without any funding or sponsorship. Dr. RJ Cerfolio is a teacher for Intuitive, C-SATS, Bovie, Ethicon, Covidien, Community Health 13. Services, Bard, and Myriad Genetics. O Aljuboori has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xie MR, Liu CQ, Guo MF, et al. Short-term outcomes of minimally invasive Ivor-Lewis esophagectomy for esophageal cancer. Ann Thorac Surg 2014;97:1721-7. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Weksler B, Sharma P, Moudgill N, et al. Robot-assisted minimally invasive esophagectomy is equivalent to thoracoscopic minimally invasive esophagectomy. Dis Esophagus 2012;25:403-9. [Crossref] [PubMed]
- Clark J, Sodergren MH, Purkarastha S, et al. The role of robotic assisted laparoscopy for oesophagogastric oncological resection; an appraisal of the literature. Dis Esophagus 2011;24:240-50. [Crossref] [PubMed]
- Lee L, Sudarshan M, Li C, et al. Cost-effectiveness of minimally invasive versus open esophagectomy for esophageal cancer. Ann Surg Oncol 2013;20:3732-9. [Crossref] [PubMed]
- Melvin WS, Needleman BJ, Krause KR, et al. Computer-enhanced robotic telesurgery: initial experience in foregut surgery. Surg Endosc 2002;16:1790-2. [Crossref] [PubMed]
- Cong Z, Diao Q, Yi J, et al. Esophagectomy combined with aortic segment replacement for esophageal cancer invading the aorta. Ann Thorac Surg 2014;97:460-6. [Crossref] [PubMed]
- Van Raemdonck D, Van Cutsem E, Menten J, et al. Induction therapy for clinical T4 oesophageal carcinoma; a plea for continued surgical exploration. Eur J Cardiothorac Surg 1997;11:828-37. [Crossref] [PubMed]
- Li J, Shen Y, Tan L, et al. Is minimally invasive esophagectomy beneficial to elderly patients with esophageal cancer? Surg Endosc 2015;29:925-30. [Crossref] [PubMed]
- Cerfolio RJ, Wei B, Hawn MT, et al. Robotic Esophagectomy for Cancer: Early Results and Lessons Learned. Semin Thorac Cardiovasc Surg 2016;28:160-9. [Crossref] [PubMed]
- Ripley RT, Sarkaria IS, Grosser R, et al. Pretreatment Dysphagia in Esophageal Cancer Patients May Eliminate the Need for Staging by Endoscopic Ultrasonography. Ann Thorac Surg 2016;101:226-230. [Crossref] [PubMed]
- Fang TC, Oh YS, Szabo A, et al. Utility of dysphagia grade in predicting endoscopic ultrasound T-stage of non-metastatic esophageal cancer. Dis Esophagus 2016;29:642-8. [Crossref] [PubMed]
- Kim JY, Correa AM, Vaporciyan AA, et al. Does the timing of esophagectomy after chemoradiation affect outcome? Ann Thorac Surg 2012;93:207-12. [Crossref] [PubMed]
- Lee A, Wong AT, Schwartz D, et al. Is There a Benefit to Prolonging the Interval Between Neoadjuvant Chemoradiation and Esophagectomy in Esophageal Cancer? Ann Thorac Surg 2016;102:433-8. [Crossref] [PubMed]
- Chiu CH, Chao YK, Chang HK, et al. Interval between neoadjuvant chemoradiotherapy and surgery for esophageal squamous cell carcinoma: does delayed surgery impact outcome? Ann Surg Oncol 2013;20:4245-51. [Crossref] [PubMed]
- Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 1994;220:50-2. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Canon CL, et al. Is botulinum toxin injection of the pylorus during Ivor Lewis [corrected] esophagogastrectomy the optimal drainage strategy? J Thorac Cardiovasc Surg 2009;137:565-72. Erratum in: J Thorac Cardiovasc Surg 2009;137:1581. [Crossref] [PubMed]
- Baker EH, Hill JS, Reames MK, et al. Drain amylase aids detection of anastomotic leak after esophagectomy. J Gastrointest Oncol 2016;7:181-8. [PubMed]
- Berkelmans GH, Kouwenhoven EA, Smeets BJ. World J Gastroenterol 2015;21:9118-25. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Bass CS, et al. Fast tracking after Ivor Lewis esophagogastrectomy. Chest 2004;126:1187-94. [Crossref] [PubMed]
- Wei B, Cerfolio RJ. Clinical pathway for thoracic surgery in the United States. J Thorac Dis 2016;8:S29-36. [PubMed]
- Hernandez JM, Dimou F, Weber J, et al. Defining the learning curve for robotic-assisted esophagogastrectomy. J Gastrointest Surg 2013;17:1346-51. [Crossref] [PubMed]
- de la Fuente SG, Weber J, Hoffe SE, et al. Initial experience from a large referral center with robotic-assisted Ivor Lewis esophagogastrectomy for oncologic purposes. Surg Endosc 2013;27:3339-47. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Finley DJ, et al. Combined thoracoscopic and laparoscopic robotic-assisted minimally invasive esophagectomy using a four-arm platform: experience, technique and cautions during early procedure development. Eur J Cardiothorac Surg 2013;43:e107-15. [Crossref] [PubMed]
- Dunn DH, Johnson EM, Morphew JA, et al. Robot-assisted transhiatal esophagectomy: a 3-year single-center experience. Dis Esophagus 2013;26:159-66. [Crossref] [PubMed]
- Park SY, Kim DJ, Yu WS, et al. Robot-assisted thoracoscopic esophagectomy with extensive mediastinal lymphadenectomy: experience with 114 consecutive patients with intrathoracic esophageal cancer. Dis Esophagus 2016;29:326-32. [Crossref] [PubMed]
- Boone J, Schipper ME, Moojen WA, et al. Robot-assisted thoracoscopic oesophagectomy for cancer. Br J Surg 2009;96:878-86. [Crossref] [PubMed]
- Kernstine KH, DeArmond DT, Shamoun DM, et al. The first series of completely robotic esophagectomies with three-field lymphadenectomy: initial experience. Surg Endosc 2007;21:2285-92. [Crossref] [PubMed]
- Galvani CA, Gorodner MV, Moser F, et al. Robotically assisted laparoscopic transhiatal esophagectomy. Surg Endosc 2008;22:188-95. [Crossref] [PubMed]
- Hodari A, Park KU, Lace B, et al. Robot-Assisted Minimally Invasive Ivor Lewis Esophagectomy With Real-Time Perfusion Assessment. Ann Thorac Surg 2015;100:947-52. [Crossref] [PubMed]
- Coker AM, Barajas-Gamboa JS, Cheverie J, et al. Outcomes of robotic-assisted transhiatal esophagectomy for esophageal cancer after neoadjuvant chemoradiation. J Laparoendosc Adv Surg Tech A 2014;24:89-94. [Crossref] [PubMed]
- Harrison LE, Yiengpruksawan A, Patel J, et al. Robotic gastrectomy and esophagogastrectomy: A single center experience of 105 cases. J Surg Oncol 2015;112:888-93. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer, a randomized controlled trial (ROBOT trial). Trials 2012;13:230. [Crossref] [PubMed]
- Sarkaria IS, Bains MS, Finley DJ, et al. Intraoperative near-infrared fluorescence imaging as an adjunct to robotic-assisted minimally invasive esophagectomy. Innovations (Phila) 2014;9:391-3. [Crossref] [PubMed]
- Wei B, D'Amico TA. Thoracoscopic versus robotic approaches: advantages and disadvantages. Thorac Surg Clin 2014;24:177-88. [Crossref] [PubMed]
- Ruurda JP, van der Sluis PC, van der Horst S, et al. Robot-assisted minimally invasive esophagectomy for esophageal cancer: A systematic review. J Surg Oncol 2015;112:257-65. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Starting a robotic program in general thoracic surgery: why, how, and lessons learned. Ann Thorac Surg 2011;91:1729-36. [Crossref] [PubMed]
Cite this article as: Aljuboori O, Cerfolio RJ. Robotic esophagectomy. Shanghai Chest 2018;2:36.