The role of lung ultrasound in the diagnosis of interstitial lung disease
What is interstitial lung disease (ILD)?
ILD is defined as thickening of the pulmonary interstitium (space between the capillary endothelium and the alveolar epithelium) leading to impaired gas exchange due to various causes. ILD may be idiopathic or caused by exposure to organic and inorganic substances (i.e., hypersensitivity pneumonitis and pneumoconiosis), medical conditions [i.e., connective tissue diseases (CTDs), multisystemic diseases and obstructive sleep apnea], drugs, infection and radiation therapy (1,2). The overall estimated prevalence of ILD is about 25–74/100,000 population and up to 80.9 per 100,000 in men and 67.2 per 100,000 in women (3). Diagnosis of ILD is usually made based on combination of clinical, functional, radiological and histological data. Chest X-ray (CXR) is often the first imaging test performed in ILD and British Thoracic Society recommend for high-resolution computed tomography (HRCT) if diagnosis is uncertain after CXR and clinical assessment. If diagnosis is still not clear, bronchoalveolar lavage, transbronchial lung biopsy or surgical lung biopsy may be considered (1). Complications of ILD are irreversible pulmonary fibrosis, respiratory failure, acute exacerbation, pulmonary artery hypertension, malignancy and thromboembolic disease. Prognosis of ILD varies by type of ILD.
LUS in thoracic conditions
Recently lung ultrasound (LUS) had become more and more important in evaluating a lot of thoracic conditions and pleural effusions (4-6). In addition it is useful as guidance in biopsies of chest wall masses. However, there are few data in the use of LUS to follow-up patients with an underlying pathologic lung conditions and as a screening tool to evaluate patients who a lung disease has been suspected (7).
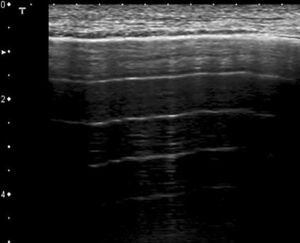
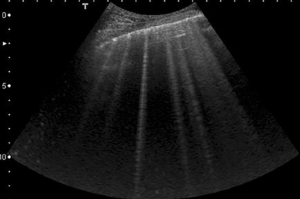
In physiological conditions the lung is a strong acoustic reflector because there is a large acoustic impedance mismatch between the chest wall and the subpleural aerated tissue. In LUS the expressions of this biological status entail the so called A-lines: the repetition of the pleural plane echo at different depths and the subpleural mirror reproductions of the parietal planes (Figure 1) (8). Occasionally, on US images of the thorax, healthy lung may produce a ring-down artifact called B-lines, (9). According to the International Consensus Conference on Pleural and Lung Ultrasound, B-lines are described as a discrete laser-like vertical hyperechoic lines arising from the pleural plane, extending to the bottom of the screen without fading and moving synchronously with the lung sliding (10). In literature B-lines have been described in pathological lung conditions, particularly when there is a diffuse parenchymal lung disease, for example lung edema, atelectasis or early pneumonia. In these conditions B-lines may reflect partial deaeration of the lung probably due to fluid accumulation (i.e., patients with heart failure or end-stage renal disease accompanied by pulmonary congestion) (Figure 2) (7,11-14) or deposition of collagen tissue (7,11). However there is not a clearly correlation between B-lines and a specific anatomical structure, rather B-lines are supposed to be correlated to the changes in the physical properties of the lung (15). Furthermore, B-lines can present as unifocal or oligofocal with variable arrangements (separate, dense or coalescent), pure or mixed with consolidations (16,17). Nowadays the presence of ≥3 B-lines between two ribs in a single scan is indicative for the presence of sonographic interstitial syndrome, a condition which may be focal, multifocal, homogeneously or non-homogeneously diffuse (10,16,18). Unfortunately both sonographic interstitial syndrome and B-lines have a low specificity: this not allow physicians to distinguish between primary pulmonary conditions and other different conditions. For example in evaluating only B-lines the physician cannot clear distinguish between a ‘‘water B-line’’ and a ‘‘connective B-line’’, moreover it is not possible to estimate how much water is superimposed on a fibrotic lung. So for differentiate between pulmonary fibrotic conditions and lung fluid accumulation we can focus not only on B-lines, but also on the pleural line and on the lung parenchyma. For example when an inflammatory interstitial syndrome is suspected, LUS can show B-lines and pleural irregularities and nodules or consolidations of the parenchyma; this give evidence for a lung pathology (Figure 3). In fact B-lines are often observed in the areas adjacent to the pneumonic consolidations and are related to pulmonary inflammatory changes (19,20).
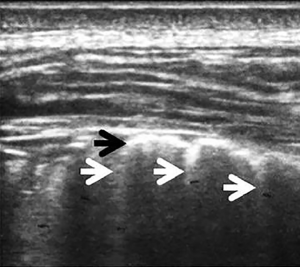
When diffuse granulomatous diseases of the lung and cryptogenic organizing pneumonia have been suspected, LUS may show subpleural micronodulations or recurring consolidations. Similarly idiopathic and secondary pulmonary fibrosis show pleural irregularities and mixed configurations of dense and coalescent B-lines, together with scattered spared or mild-diseased areas on LUS (corresponding to subpleural tissue distortion that explain abnormalities showed on CT imaging) (Figure 4) (19,20). However there is no consensus on the specific diagnostic criteria in defining ILD by LUS (21) and to date most of studies calculated a total B-line score (TBLS): a complete count of B-lines on the entire thoracic surface, defining ILD as a TBLS >5 or >10 (see below) (22-25).
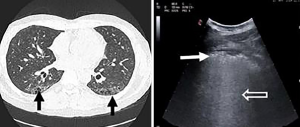
Importantly, Delle Sedie et al. showed there is not a single transducer to use: probes with frequencies ranging between 3 and 3.5 MHz and frequencies between 5 and 7.5 MHz can be equally used in detecting ILD (26).
Here we discuss use of LUS in ILD.
LUS in ILD
Nowadays there are an increasing number of studies that showed the utility of LUS in ILD, most of them in rheumatological diseases, as we discuss in next paragraph. Here we explain use of LUS in non rheumatological ILD conditions.
Singh et al. (27), in a single center study, tried to differentiate between cardiogenic pulmonary edema (CPE) and noncardiogenic alveolar interstitial syndrome (NCAIS) using M-Mode US with a P21 phased array probe and a L30 linear vascular probe. Excluding subjects with an overlapping diagnosis of CPE and NCAIS, they enrolled 43 patients with previous demonstrated ILD and divided them into three groups: NCAIS group, CPE group and a control group. Afterwards they analyzed 308 lung zones for pleural line morphologic features and 304 lung zones for subpleural morphologic features. On M-mode US they found a fragmented pleural line and a vertical subpleural pattern in most patients with NCAIS, while most patients with CPE have a continuous pleural line and a vertical subpleural pattern. The sensitivity and the specificity to distinguish between the two conditions was 67.8% and 89.2% respectively, with k value of agreement between interpreters ≥94.6%. Differently Santana et al. (28) showed the usefulness of LUS in the diagnosis of ILD identifying decreased diaphragmatic mobility and diaphragm thickening. Matching for age, gender, body mass index and smoking status, they enrolled 40 ILD patients and 16 healthy volunteers who underwent LUS examination and pulmonary functional test. Initially LUS were performed using a 2–5 MHz convex probe on M-mode: they insonated the anterior subcostal region (space between the midclavicular and anterior axillary lines) and measured and recorded the average value of three consecutive measurements of diaphragm mobility both during quiet breathing and deep breathing. Afterwards by B-Mode with a 6–13 MHz linear transducer positioned near the costophrenic angle (space between the right anterior and medial axillary lines) they measured diaphragm thickening and recorded the distance from the pleural line to the peritoneal line. Compared to controls, they found ILD patients had a reduced mobility in diaphragm mobility just during deep breathing (7.62±1.44 vs. 4.46±1.73 cm; Pvs. 0.32±0.08 cm; P
Other studies tried to compare LUS as diagnostic tool of ILD. For example, Vizioli et al. (29) compared LUS and CXR for diagnosis of ILD using HRCT as the gold standard in 104 patients with ILD have been suspected. Moreover, in confirmed ILD patients, they compared the accuracy of different echographic diagnostic criteria. Using linear (5–8 MHz) and convex (2–5 MHz) probe, they analyzed 12 chest areas per side for each patient: 6 per side to study the anterolateral lung fields (patient in supine decubitus position) and 6 per side to study the posterior lung fields (patient in the seated position). According to the recommendations of the International Consensus Conference on Lung Ultrasound (10), they recorded and evaluated for each area B-line distribution (focal or diffuse), the sonographic pattern (homogeneous or not) and the presence and characterization of pleural line abnormalities (irregularity, fragmentation, subpleural consolidations and swelling). Then they calculated TBLS (≥10 or ≥5) and tried to make a simplified score considering LUS positive for ILD if ≥5 B-lines were present in ≥3 chest areas (not necessarily contiguous ones). Using HRCT as the gold standard, CXR vs LUS showed lower sensitivity (0.28–0.67 CXR vs. 0.81–1.00 LUS), but higher specificity (0.81–1.00 CXR vs. 0.53–0.89 LUS), suggesting that CXR and LUS may be complementary in diagnosis. Using LUS for diagnosis of ILD, the simplified score had the same sensitivity as TBLS (0.84–0.99), but higher in specificity (0.69–0.90 LUS vs. 0.54–0.79 for TBLS ≥10 and vs. 0.40–0.67 for TBLS ≥5).
In 2009 Volpicelli at al. and in 2012 Testa et al. show the usefulness of LUS in detecting viral pneumonia. In this cohort of patients LUS gives a particular pattern with spared areas strongly suggestive for viral pneumonia. In fact in viral pneumonia LUS findings had a good correlation with CT scans findings (30,31). Likewise, Asano et al. (32) found usefulness of LUS in diagnosis of interstitial pneumonia. According to the American Thoracic Society/European Respiratory Society Guidelines, 40 patients with interstitial pneumonia underwent CXR, CT, HRCT, LUS (using a 7.5 MHz linear probe), blood tests and clinical function tests to compare the US finding with others features of interstitial pneumonia. With the patients in seated position, the authors insonated both the right and left sides of posterior chest wall studying paravertebral, subscapular and posterior axillary lines sites of both the subscapular and lung base areas (12 locations overall). So they evaluated B-lines and pleural abnormalities (thickening and irregularities) finding a positive correlation with total numbers of B-lines and clinical, radiological and serological activity of the interstitial disease. Moreover, as expected, there was a negative correlation between numbers of B-lines and forced vital capacity, diffusion capacity for carbon monoxide and SpO2 level after the 6-minute walk test.
Other studies show a direct correlation between an increased number of B-lines, an irregular and/or blurred pleural line and the presence or absence of white lung by LUS and increased systolic pulmonary arterial pressure as expression of pulmonary hypertension. For example Zhu et al. found that a LUS score (a combination of B-lines, pleural irregularities and presence/absence of white lung) >16 points predicts pulmonary hypertension (using a cut-off of systolic pulmonary arterial pressure >36 mmHg) in ILD patients with a sensitivity of 85.2% and a specificity of 80.3% (33).
By contrast Zheng et al. reported that only a score of B-lines >4 on LUS can predict elevated systolic pulmonary pressure (>30 mmHg) with 89.5% sensitivity, 85.0% specificity and 87.2% accuracy (34).
LUS in CTD-associated ILD
ILD is a frequent parenchymal manifestation of CTDs and to date HRCT is the gold standard to diagnose CTD-ILD, however patients cannot repeat HRCT very often because is associated with high radiation exposure. Since LUS is a non-invasive and nonionizing modality, rheumatologists and internists tried to use it to assess and follow-up CTD-ILD. The meta-analysis by Song et al. supported these data showing that the number of B-lines had a good correlation with the HRCT fibrosis pattern and good diagnostic accuracy in terms of sensitivity (91.5%) and specificity (81.3%) (35). Moreover an exhaustive overview has been proposed in 2016 by Gutierrez et al. who systematically evaluated the literature published on the topic between January 2000 and December 2015 (36).
LUS in systemic sclerosis (SSc)
Nowadays most studies of LUS in rheumatological disease has been made in patients with SSc. Gargani et al. (22) showed LUS like a powerful tool in evaluating interstitial pulmonary fibrosis (IPF). In 33 consecutive SSc (both limited and diffuse type) patients, they compared LUS and chest HRCT showing a high correlation between HRCT and LUS finding (in terms of total number of B-lines) in quantification of IPF. Moreover, as expected, B-lines, were more frequent in the diffused form than in the limited form of SSc. Similar results on SSc patients have been showed by Delle Sedie et al. (26) on SSc patients they found a good sensitivity (85%) and specificity (70%) of LUS findings compared to the chest HRCT findings. The pilot study of Barskova et al. (23) gave us important data on the role of LUS in the diagnosis of early SSc. The authors enrolled 58 consecutive patients with SSc: 32/58 patients (55%) had very early SSc. All patients underwent chest HRCT and LUS. The reported overall concordance between LUS and HRCT in assessment of IPF was 83%. Importantly, analyzing discordant cases, the authors find that all positive cases were reported by LUS, which gave a sensitivity and negative predictive value of 100% in detecting SSc and early SSc. In order to facilitate the correlation with chest HRCT finding and to establish IPF severity, Tardella et al. (25) created a semiquantitative score of B-lines on LUS and compared it with HRCT score. They enrolled 34 consecutive patients with different CTDs who underwent LUS (on the anterior, medial and posterior chest areas), HRCT and lung functional tests. By LUS, IPF involvement might be grade 0 = normal (50 B-lines). So they compared HRCT score with the LUS score and showed a significant linear correlation. Moreover they found a positive correlation between LUS B-lines and values of diffusing capacity of the lungs for carbon monoxide test. Both results correlated with severity of IPF. Unlike Tardella, but as previous studies, Gutierrez et al. (37) tried to use a simplified LUS assessment of ILD. They enrolled 36 patients with CTDs and evaluating B-lines on LUS and chest HRCT findings (used as gold standard). The authors performed LUS in evaluating anterior [2nd intercostal spaces (IS) along parasternal lines and 4th IS along midclavicular, axillary and midaxillary lines] and posterior (along the paravertebral, subscapular and posterior axillary lines) chest areas. Compared to HRCT, they found a good correlation between HRCT findings and the simplified LUS B-line assessment in the diagnosis of IPF. Likewise Tardella, Mohammadi et al. (38) tried to test a reduced LUS score (in evaluating B-lines) again in the diagnosis of IPF in SSc patients. In evaluating B-lines on LUS, differently to Tardella, they insonated anterior (4th IS along the midclavicular line), lateral (4th IS along the anterior axillary and midaxillary line) and posterior (8th IS along the anterior, subscapular, and posterior axillary lines) chest areas and compared US findings to chest HRCT findings (considered as the gold standard). Compared to HRTC, reduced LUS score showed a good sensitivity (74%), specificity (88%) and positive (95%) and negative (52%) predictive values in establishing the severity of pulmonary involvement. Differently to previous studies described in this paragraph, Moazedi-Fuerst et al. (39), in order to evaluate the reliability of LUS in the diagnosis of SSc patients, using chest HRCT as the gold standard, evaluated not only B-lines but also pleural irregularities on chest US. In 25 SSc patients and in a similar number of healthy patients, they found both B-lines and pleural thickening on LUS in 44% vs 7% of SSc patients compared to healthy subjects. Two other studies correlate pleural irregularities and B-lines in CTDs patients. Hasan et al. (40), in 61 patients with different CTDs, showed that the severity of ILD had a positive correlation between B-lines on LUS and the findings on chest HRCT in terms of ground glass opacity, extensive fibrosis and honey combing. Likewise Sperandeo et al. (20) enrolled 175 SSc patients who underwent LUS examination and chest HRCT. They found a good correlation between pleural and subpleural irregularities and nodules on LUS and the HRCT findings.
In 2016, Buda et al. (41) correlate both LUS findings and severity of ILD and LUS findings and HRCT. The authors enrolled 52 patients with demonstrated ILD and 50 healthy subjects and establish their own criteria of ILD on LUS in terms of pleural irregularities and B-lines. Compared to chest HRCT findings, they found a positive correlation between pleural irregularities (fragmented, blurred and/or thickened pleural line and presence/absence of white lung) and the progression of ILD. Importantly they also found that, at the start of disease, pleural irregularities were most often in the lower fields of the lungs and progressively there was a spreading in middles and upper fields of the lungs with the progression of ILD.
Recently Gigante et al. (42), in 39 SSc patients, correlate US, chest HRCT and pulmonary function tests (PFTs) findings. They found that number of B-lines had a positive correlation to HRCT score and a negative correlation to DLCO values.
LUS in other rheumatic disorders
In 2013, Aghdashi et al. (43), evaluating B-lines in 31 consecutive patients with potential rheumatoid lung involvement, found a sensitivity of 74%, a specificity of 88% and a positive and negative predictive value of 95% and 52% respectively, in comparing LUS with chest HRCT. Cogliati et al. (24) enrolled 39 patients with rheumatoid arthritis (RA) in order to measure the accuracy of LUS, compared to chest HRCT, in the diagnosis of ILD using both standard equipment and a pocketsize US device (PS-USD) (tough the latter was mostly used as a screening tool). They assumed a positive examination when B-lines score was >10, so they ensonated 28 anterior and 44 posterior chest areas and recorded B-lines. Using standard US, the authors found a sensitivity of 92% and a specificity of 56% compared to HRCT (with a positive correlation between B-line score and HRCT score). Using PS-USD vs. HRCT sensitivity was 89% and specificity was 50%.
Moazedi-Fuerst et al. (44), in a different study from the previous one, hypothesized the usefulness of LUS in detecting lung abnormalities in RA patients who did not present any clinical signs or symptoms of IPF. They enrolled 64 consecutive RA patients and 40 healthy volunteers who underwent both LUS (in order to evaluate pleural abnormalities and lung parenchyma) and chest HRCT. Compared to the latter, LUS showed B-lines and pleural nodules in 28% of RA patients, but also showed 7% of abnormalities in the healthy volunteers, not allowing to use it in an early stage of disease. In 2015 the same authors (45) compared LUS to chest HRCT as diagnostic tool of IPF. Moreover they tried to find a possible correlation between the underlying rheumatological disease and a specific US pattern. The study enrolled 45 patients with IPF [25 with RA, 14 with SSc and 6 with systemic lupus erythematosus (SLE)] and 40 healthy subjects. In performing LUS, they insonated 9 chest areas for each side (divided into anterior, lateral and posterior) and evaluated pleural and subpleural abnormalities and B-lines which resulted more frequent in IPF patients than healthy patients, as expected. Moreover the authors found a significant different pattern of LUS abnormalities in different IPF disease. In fact in the group of IPF patients Moazedi-Fuerst et al. recorded subpleural nodes in 100% of the RA patients vs. 22% of the SSc patients (P=0.003) and vs. 50% of the SLE patients (P=0.049). Furthermore an irregular and thickened pleural line (>3 mm) was showed in 100% of SSc and SLE patients with IPF vs. 86% of IPF patients suffering from RA.
Likewise, Pinal- Fernandez et al. (46), in a study with 37 patients with a rheumatological disease (21 with antisynthetase syndrome, of which 2/21 without IPF and 16 with SSc, of which 6 without IPF) showed a positive correlation between LUS findings (again in terms of pleural irregularities and B-lines) and Warrick semiquantitative score at HRCT both in antisynthetase syndrome and SSc patients.
Lastly Vasco et al. (47) showed a positive correlation between B-lines at LUS examination and pulmonary fibrosis at chest HRCT in a small study with 13 Sjögren’s syndrome patients. The authors showed LUS sensitivity and specificity of 100% and 89% respectively in detecting ILD.
Table 1 summarizes and compares CTD-ILD studies discussed in rheumatological diseases.
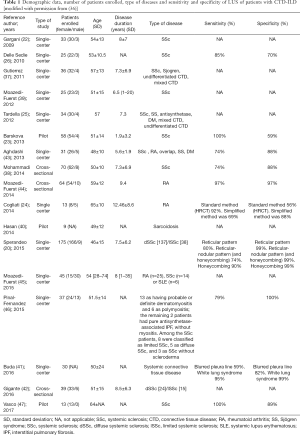
Full table
Conclusions
Although there is not a standardized approach for the LUS examination in ILD, most studies included a small cohort of patients and there is potential bias linked to changes in lung parenchyma over time, this review showed how US might have the necessary attributes to facilitate the best clinical practice in the detection of ILD. Applications of this imaging technology are still being developed and further opportunities with ongoing research strategies are likely to arise, especially to test its concurrent validity, reliability and responsiveness in multicenter studies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008;63 Suppl 5:v1-58. [Crossref] [PubMed]
- Antin-Ozerkis D, Rubinowitz A, et al. Interstitial lung disease in the connective tissue diseases. Clin Chest Med 2012;33:123-49. [Crossref] [PubMed]
- Brown KK. Chronic cough due to chronic interstitial pulmonary diseases: ACCP evidence-based clinical practice guidelines. Chest 2006;129:180S-185S. [Crossref] [PubMed]
- Sperandeo M, Rotondo A, Guglielmi G, et al. Transthoracic ultrasound in the assessment of pleural and pulmonary diseases: use and limitations. Radiol Med 2014;119:729-40. [Crossref] [PubMed]
- Ianniello S, Piccolo CL, Buquicchio GL, et al. First-line diagnosis of paediatric pneumonia in emergency: lung ultrasound (LUS) in addition to chest-X-ray (CXR) and its role in follow-up. Br J Radiol 2016;89:20150998. [Crossref] [PubMed]
- Wang L, Guan X, Chen M, et al. Clinical value of lung ultrasound in the late goal-directed fluid removal in critically ill patients underwent fluid resuscitation. Zhonghua Yi Xue Za Zhi 2016;96:1359-63. [PubMed]
- Lichtenstein D, Mézière G, Biderman P, et al. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med 1997;156:1640-6. [Crossref] [PubMed]
- Soldati G, Demi M, Inchingolo R, et al. On the Physical Basis of Pulmonary Sonographic Interstitial Syndrome. J Ultrasound Med 2016;35:2075-86. [Crossref] [PubMed]
- Gustafsson M, Alehagen U, Johansson P. Imaging Congestion With a Pocket Ultrasound Device: Prognostic Implications in Patients With Chronic Heart Failure. J Card Fail 2015;21:548-54. [Crossref] [PubMed]
- Volpicelli G, Elbarbary M, Blaivas M, et al. International Liaison Committee on Lung Ultrasound (ILC-LUS) for the International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012;38:577-91. [Crossref] [PubMed]
- Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound 2014;12:25. [Crossref] [PubMed]
- Agricola E, Bove T, Oppizzi M, et al. “Ultrasound comet-tail images”: a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest 2005;127:1690-5. [Crossref] [PubMed]
- Jambrik Z, Monti S, Coppola V, et al. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol. 2004;93:1265-70. [Crossref] [PubMed]
- Mallamaci F, Benedetto FA, Tripepi R, et al. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging 2010;3:586-94. [Crossref] [PubMed]
- Soldati G, Inchingolo R, Smargiassi A, et al. Ex vivo lung sonography: morphologic-ultrasound relationship. Ultrasound Med Biol 2012;38:1169-79. [Crossref] [PubMed]
- Smargiassi A, Inchingolo R, Soldati G, et al. The role of chest ultrasonography in the management of respiratory diseases: document II. Multidiscip Respir Med 2013;8:55. [Crossref] [PubMed]
- Soldati G, Testa A, Silva FR, et al. Chest ultrasonography in lung contusion. Chest 2006;130:533-8. [Crossref] [PubMed]
- Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound 2008;6:16. [Crossref] [PubMed]
- Reissig A, Kroegel C. Transthoracic sonography of diffuse parenchymal lung disease: the role of comet tail artifacts. J Ultrasound Med 2003;22:173-80. [Crossref] [PubMed]
- Sperandeo M, De Cata A, Molinaro F, et al. Ultrasound signs of pulmonary fibrosis in systemic sclerosis as timely indicators for chest computed tomography. Scand J Rheumatol 2015;44:389-98. [Crossref] [PubMed]
- Reissig A, Copetti R. Lung ultrasound in community-acquired pneumonia and in interstitial lung diseases. Respiration 2014;87:179-89. [Crossref] [PubMed]
- Gargani L, Doveri M, D’Errico L, et al. Ultrasound lung comets in systemic sclerosis: a chest sonography hallmark of pulmonary interstitial fibrosis. Rheumatology (Oxford) 2009;48:1382-7. [Crossref] [PubMed]
- Barskova T, Gargani L, Guiducci S, et al. Lung ultrasound for the screening of interstitial lung disease in very early systemic sclerosis. Ann Rheum Dis 2013;72:390-5. [Crossref] [PubMed]
- Cogliati C, Antivalle M, Torzillo D, et al. Standard and pocket-size lung ultrasound devices can detect interstitial lung disease in rheumatoid arthritis patients. Rheumatology (Oxford) 2014;53:1497-503. [Crossref] [PubMed]
- Tardella M, Gutierrez M, Salaffi F, et al. Ultrasound in the assessment of pulmonary fibrosis in connective tissue disorders: correlation with high-resolution computed tomography. J Rheumatol 2012;39:1641-7. [Crossref] [PubMed]
- Delle Sedie A, Doveri M, Frassi F, et al. Ultrasound lung comets in systemic sclerosis: a useful tool to detect lung interstitial fibrosis. Clin Exp Rheumatol 2010;28:S54. [PubMed]
- Singh AK, Mayo PH, Koenig S, et al. The Use of M-Mode Ultrasonography to Differentiate the Causes of B Lines. Chest 2018;153:689-96. [Crossref] [PubMed]
- Santana PV, Prina E, Albuquerque ALP, et al. Identifying decreased diaphragmatic mobility and diaphragm thickening in interstitial lung disease: the utility of ultrasound imaging. J Bras Pneumol 2016;42:88-94. [Crossref] [PubMed]
- Vizioli L, Ciccarese F, Forti P, et al. Integrated use of lung ultrasound and chest X-ray in the detection of interstitial lung disease. Respiration 2017;93:15-22. [Crossref] [PubMed]
- Testa A, Soldati G, Copetti R, et al. Early recognition of the 2009 pandemic influenza A (H1N1) pneumonia by chest ultrasound. Crit Care 2012;16:R30. [Crossref] [PubMed]
- Volpicelli G, Frascisco MF. Sonographic detection of radio-occult interstitial lung involvement in measles pneumonitis. Am J Emerg Med 2009;27:128.e1-128.e3. [Crossref] [PubMed]
- Asano M, Watanabe H, Sato K, et al. Validity of Ultrasound Lung Comets for Assessment of the Severity of Interstitial Pneumonia. J Ultrasound Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Zhu WW, Li YD, Li H, et al. Integrative Cardiopulmonary Ultrasound for Interstitial Lung Disease Assessment: Correlation between Lung Ultrasound Performance and Cardiac Involvement. Ultrasound Med Biol 2017;43:744-52. [Crossref] [PubMed]
- Zheng XZ, Zheng Q, Zhou J, et al. B-lines in assessment of pulmonary hypertension in patients with interstitial lung diseases: feasibility of transthoracic lung sonographic signs. J Ultrasound Med 2015;34:1669-75. [Crossref] [PubMed]
- Song G, Bae SC, Lee YH. Diagnostic accuracy of lung ultrasound for interstitial lung disease in patients with connective tissue diseases: a meta-analysis. Clin Exp Rheumatol 2016;34:11-6. [PubMed]
- Gutierrez M, Gomez-Quiroz LE, Clavijo-Cornejo D, et al. Ultrasound in the interstitial pulmonary fibrosis. Can it facilitate a best routine assessment in rheumatic disorders? Clin Rheumatol 2016;35:2387-95. [Crossref] [PubMed]
- Gutierrez M, Salaffi F, Carotti M, et al. Utility of a simplified ultrasound assessment to assess interstitial pulmonary fibrosis in connective tissue disorders—preliminary results. Arthritis Res Ther 2011;13:R134. [Crossref] [PubMed]
- Mohammadi A, Oshnoei S, Ghasemi-rad M. Comparison of a new, modified lung ultrasonography technique with high-resolution CT in the diagnosis of the alveolo-interstitial syndrome of systemic scleroderma. Med Ultrason 2014;16:27-31. [Crossref] [PubMed]
- Moazedi-Fuerst FC, Zechner PM, Tripolt J, et al. Pulmonary echography in systemic sclerosis. Clin Rheumatol 2012;31:1621-5. [Crossref] [PubMed]
- Hasan AA. Makhlouf HA B-lines: Transthoracic chest ultrasound signs useful in the assessment of interstitial lung diseases. Ann Thorac Med 2014;9:99-103. [Crossref] [PubMed]
- Buda N, Piskunowicz M, Porzezinska M, et al. Lung ultrasonography in the evaluation of interstitial lung disease in systemic connective tissue diseases: criteria and severity of pulmonary fibrosis—analysis of 52 patients. Ultraschall Med 2016;37:379-85. [PubMed]
- Gigante A, Rossi Fanelli F, Lucci S, et al. Lung ultrasound in systemic sclerosis: correlation with high-resolution computed tomography, pulmonary function tests and clinical variables of disease. Intern Emerg Med 2016;11:213-7. [Crossref] [PubMed]
- Aghdashi M, Brofeh B, Mohammadi A. Diagnostic performances of high resolution trans-thoracic lung ultrasonography in pulmonary alveoli-interstitial involvement of rheumatoid lung disease. Int J Clin Exp Med 2013;6:562-6. [PubMed]
- Moazedi-Fuerst FC, Kielhauser SM, Scheidl S, et al. Ultrasound screening for interstitial lung disease in rheumatoid arthritis. Clin Exp Rheumatol 2014;32:199-203. [PubMed]
- Moazedi-Fuerst FC, Kielhauser S, Brickmann K, et al. Sonographic assessment of interstitial lung disease in patients with rheumatoid arthritis, systemic sclerosis and systemic lupus erythematosus. Clin Exp Rheumatol 2015;33:S87-91. [PubMed]
- Pinal-Fernandez I, Pallisa-Nuñez E, Selva-O’Callaghan A, et al. Pleural irregularity, a new ultrasound sign for the study of interstitial lung disease in systemic sclerosis and antisynthetase syndrome. Clin Exp Rheumatol 2015;33:S136-41. [PubMed]
- Vasco PG, de Luna CG, Garrido IM, et al. Assessment of interstitial lung disease in Sjogren's syndrome by lung ultrasound: a pilot study of correlation with high-resolution chest tomography. Intern Emerg Med 2017;12:327-31. [Crossref] [PubMed]
Cite this article as: Falcetta A, Leccardi S, Testa E, Papaleo F, Fenoglio L, Melchio R. The role of lung ultrasound in the diagnosis of interstitial lung disease. Shanghai Chest 2018;2:41.