
Video-assisted thoracoscopic surgery in the management of mesothelioma
Video-assisted thoracoscopic surgery (VATS) in the management of mesothelioma
Malignant pleural mesothelioma (MPM) is almost always a terminal diagnosis. Other than for rare cases of localised MPM which has been completely resected in combination with chemotherapy/radiotherapy, there is no treatment available that offers the possibility of a cure, but only palliative treatments that may in some cases also improve prognosis. The role of surgery in the management of mesothelioma is controversial, with a paucity of high quality evidence to support management decisions. In this review, the role of VATS in the management of MPM is considered.
MPM
MPM is the commonest primary tumour of the pleura. It is strongly associated with exposure to asbestos, usually several decades prior to clinical presentation (1). Although the incidence continues to increase, it is anticipated that this will begin to fall in the future as asbestos use falls (2,3). Diagnosis is often late because symptoms are rather non-specific, particularly at early stages of the disease. The majority of patients present with advanced disease. The commonest symptoms are dyspnoea and chest discomfort. Dyspnoea may be a result of pleural fluid accumulation or lung encasement by tumour, which results in decreased chest wall expansion (4). There are three types of MPM histologically: epithelioid (50–70%) and sarcomatoid (10–20%), with the remaining patients having a mixed, biphasic pattern (5). In view of the advanced stage at diagnosis, patients managed with best supportive care alone have a median survival following diagnosis of between 6–18 months with <5% patients surviving >5 years (4,6). There is now evidence from clinical trials supporting the use of platinum-containing chemotherapy demonstrating survival advantage and this is now considered the minimum standard of care for patients with MPM and a satisfactory performance status (7,8).
Surgery for MPM
The role of surgery as a treatment option remains controversial as a result of studies demonstrating marginal, if any, overall benefit from surgery with significant associated morbidity and mortality (9). In many selected cases there may be advantage but unfortunately this is hidden in the overall results. Identifying those most likely to benefit is difficult.
Video assisted thoracoscopic surgery for MPM
With the evolution of thoracic surgery to more minimally invasive VATS procedures, the role of surgery in the management of MPM has been potentially expanded. Although extrapleural pneumonectomy (EPP) which involves en-bloc resection of the lung and surrounding pleura, ipsilateral hemidiaphragm and ipsilateral pericardium followed by reconstruction, is typically an open procedure, there are two case reports of EPP being performed by VATS (10,11). It is worth noting that the operations took in excess of 14 hours and this is unlikely to become a widely performed procedure.
More commonly, VATS can play a role in the diagnosis of MPM, control of the pleural space, debulking and decortication to reinflate the lung.
VATS pleural biopsy
Confirming the diagnosis of MPM is important to guide on-going management but also has for securing compensation that is available to patients in some countries. Diagnostic yield from cytological analysis of pleural fluid is low, CT guided needle biopsies are better but are still often equivocal (1,4,12). Greater amounts of tissue can be retrieved by thoracoscopy without general anaesthetic (so-called ‘medical thoracoscopy’) but this may not be suitable for all patients (13).
Performing pleural biopsy by VATS offers the advantage of visualisation and targeted sampling of representative areas from different sites within the pleural cavity. Large biopsies can be taken, significantly increasing the likelihood of confirming the diagnosis (14). The procedure allows for complete drainage of any pleural effusion and exploration of the entire thoracic cavity permitting assessment of the extent of disease burden. Furthermore, at the end of the procedure, positive-pressure ventilation under vision will enable assessment of the extent of lung re-expansion following effusion drainage to guide ongoing management (12). VATS pleural biopsy is a very well-tolerated procedure, with no patient awareness and typically a short length of hospital stay.
VATS talc pleurodesis
If full lung expansion can be achieved following drainage of pleural effusion, the goal is to achieve pleurodesis which aims to obliterate the pleural cavity to prevent re-accumulation of pleural fluid and therefore prevent recurrence of dyspnoea. The success of achieving pleurodesis is dependent on the extent of pleural disease, the rapidity of fluid re-accumulation and the capacity of the lung to fully expand (15). The most popular agent used to achieve pleurodesis is sterile talc. This can be introduced as slurry through a chest drain, or insufflated at surgery. The has been a report suggesting that in terms of efficacy there is no difference between surgical and bedside talc pleurodesis (15). However, we would argue that the advantage of performing this by VATS is that re-expansion of the lung can be tested and confirmed prior to administration and this can be performed at the same time as biopsy acquisition. Furthermore, the significant pain that is experienced by some patients following talc slurry introduction is avoided or reduced by performing this under general anaesthesia (15).
Large studies have been performed examining the outcomes of VATS talc pleurodesis in MPM. One such study by Rena et al. examined outcomes following VATS talc poudrage in 172 patients (16). By 3 months, 49% patients had a complete response with no re-accumulation of pleural effusion and the majority of those were found to still have a full response at 12 months. The impact of achieving sustained pleurodesis is demonstrated by the fact that pleural fluid recurrence at 3 months was one of the prognostic factors identified by multivariate analysis to be predictive of poor outcome. Median survival of those with sustained pleurodesis was 19.5 months compared with 9 months for those without. This resulted in a 2-year disease specific survival of ~30% which is comparable to that achieved with the radical surgical options described above. The high failure rate to achieve sustained pleurodesis is common across other studies examining talc pleurodesis in MPM (15). It is observed that failure rate is higher than that observed in pleural effusion associated with other malignancies (17). It is thought that this may reflect the MPM disease process affecting diffusely the parietal and visceral pleura, impairing the ability to achieve full re-expansion of the lung and apposition of visceral and parietal pleura, but also the paucity of normal mesothelial cells may be important (18,19).
VATS partial pleurectomy and decortication
The above observation that patients in whom pleurodesis is achieved have acceptable survival and quality of life led some surgeons to offer VATS partial pleurectomy and decortication (VATS-P/D) to patients with MPM in order to release trapped lung and permit re-expansion and a chance of achieving pleurodesis (20). At our centre a decortication of visceral pleura is performed releasing the lung from entrapment. If the lung can then re-expand such that the decorticated regions appear likely to reach the chest wall, a parietal pleurectomy is performed to both further debulk the disease and also facilitate pleurodesis (Figures 1,2 and video: https://youtu.be/g9z_atuQ3Jk). In our practice we feel it is important to ensure that the lung can come up against the area where the parietal decortication has taken place with the aim of tamponading bleeding and also achieving apposition which is a pre-requisite for pleurodesis.
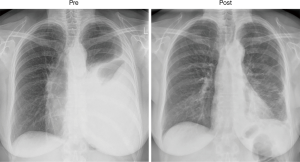
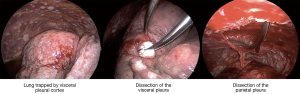
One study examining outcomes following VATS-P/D reported on 79 consecutive patients with advanced MPM (21). Partial pleurectomy and decortication was achieved in 51 patients, the remaining patients in whom it was deemed not possible had pleural biopsy alone. Those patients having VATS-P/D had significantly greater incidence of post-operative air leak and therefore longer length of hospital stay. There was one perioperative death in both groups. Patient survival though was improved with a median survival of 416 days compared to 127 in the remaining patients having only pleural biopsy (P<0.001).
The significant impact on survival may be a combination of two factors:
- closure of the pleural space—as seen in the VATS pleurodesis patients improved survival is observed. This may reflect reduced incidence of subsequent pleural effusion with a longer maintenance of performance status;
- debulking the tumour and achieving some local control—there is seems to be a long tumour doubling time in MPM of up to 700 days (22). Thus there may be significant time following such debulking before a significant volume of tumour re-accumulates.
Another study compared outcomes following VATS-P/D with the radical surgical procedures—EPP and P/D (23). The authors reported on 208 consecutive patients. Of these 112 underwent EPP, 29 P/D and the remaining 67 had VATS-P/D. The authors reported a mean survival of 11.5 months in the EPP group and 14 months in the VATS-P/D group. There was a significantly longer hospital stay in the EPP group who suffered significantly greater morbidity—60% experienced complications. The authors compared 30-day mortality for patients >65 in each group and found a 23% rate in the EPP group compared to 7.1% in the VATS-P/D group. The patients in the VATS-P/D group had improved quality of life with 58% reporting improvement in pain and 83% reporting improvement in dyspnoea. The authors conclude that on the basis of their findings that VATS-P/D is an effective palliative treatment for patients with MPM and trapped lung—particularly in older patients for whom radical surgery may not be appropriate.
MesoVATS
To further investigate the value of VATS-P/D, the MesoVATS randomised controlled trial was performed in the UK, comparing VATS-P/D with talc pleurodesis (either talc slurry through a chest drain or talc poudrage by VATS) with the aim of assessing both efficacy and cost (24). The primary endpoint was 1 year survival after randomisation. A total of 196 patients were recruited with 87 patients randomised to undergo VATS-P/D and 88 to undergo talc pleurodesis. Unsurprisingly surgical complications were more common in the surgical VATS-P/D group (31% compared with 14%, P=0.019), and median length of hospital stay was longer (7 vs. 3 days, P<0.0001). VATS-P/D was therefore more expensive. Median survival was similar with VATS-P/D at 13.1 months compared with 13.5 months in the talc pleurodesis group. The proportion of patients with resolved pleural effusion was significantly higher in the VATS-P/D group at 6 months (77% vs. 57%, P=0.028). Similarly, quality of life assessment revealed significant differences in favour of VATS-P/D from 6 months postoperatively. The authors concluded that VATS-P/D does not improve overall survival in patients with MPM pleural effusion and because it was associated with more complications, longer hospital stay and was more expensive that talc pleurodesis was preferable.
This was a very controversial conclusion since there were some cases in both groups that fared much better.
This study has put into question the benefit of VATS-P/D for patients with MPM. However, there are critics of the MesoVATS study who highlight that recruitment occurred over a prolonged period (10 years) during which significant changes in clinical practice occurred, particularly in the field of enhanced recovery and discharge with ambulatory drains and the move towards VATS talc poudrage becoming more popular than talc slurry (25). However, the result of this study has impacted on clinical practice and referrals for VATS-P/D appear to have fallen.
Mesotrap study
It is accepted that talc administration is unlikely to be effective if the lung does not reach the chest wall even after full drainage of the effusion. It is also considered reasonable that patients with trapped lung who successfully undergo VATS-P/D do better than patients who stay with trapped lung. Because of this the MESOTRAP study was developed and commenced recruitment in 2017.
MESOTRAP is a pilot clinical trial and feasibility study comparing video-assisted thoracoscopic partial pleurectomy/decortication with indwelling pleural catheter in patients with trapped lung due to MPM designed to address recruitment and randomisation uncertainties and sample size requirements for a phase III trial. (https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/mesotrap-pilot-clinical-trial-and-feasibility-study/). It is unlikely that any results will be available before 2020.
Conclusions
Putting all of the above findings together, it is clear that VATS is a valuable tool in the palliative management of patients with MPM. Firstly, as a diagnostic tool providing tissue for histological diagnosis which is essential for patients with malignant pleural effusion, particularly considering the compensation that is available for patients with MPM in many countries due to industrial exposure. Secondly, VATS is useful for draining pleural effusion to facilitate lung re-expansion and achieve pleurodesis with talc poudrage—which has been shown to contribute to prolonged survival and improved quality of life. For patients with trapped lung with a low disease burden VATS partial pleurectomy and decortication could be performed but the value of that has been put into question by the overall conclusions of the MesoVATS study making the role of VATS-P/D now unclear. Mesotrap is a new study which seeks to determine if VATS-P/D has a role.
Currently in patients suitable for VATS-PD on the basis of low disease burden, it may be considered an alternative to open P/D as the latter appears to have much higher morbidity with no clear survival advantage. However, some groups take an opposing view. For the maximal benefits of VATS-P/D being realized it is important that full lung expansion is achieved with no residual pleural space. The results of the ongoing study MESOTRAP study may inform and alter guidance on the utility of VATS-P/D in the future.
The use of VATS should be part of multimodal therapy with platinum based chemotherapy. The role of radiotherapy—particularly intervention site irradiation, will soon be clarified with publication of the results of the PIT trial. The management strategy of each patient with MPM should be discussed by the oncology multidisciplinary team, involving as a minimum: respiratory physicians, thoracic surgeons, oncologists, palliative care physicians and specialist nurses.
Key messages
VATS is safe and well tolerated and can be performed under sedation or general anaesthesia.
VATS can be performed for diagnosis, palliation of effusions and in some cases to debulk and decorticate the lung as part of the management of trapped lung.
VATS partial pleurectomy decortication (VATS-P/D) may have a role in selected patients and appears to have lower morbidity than more extensive surgery such as open pleurectomy decortication or the more morbid procedure of EPP.
VATS partial pleurectomy decortication should be considered as a first-line option in patients with low disease burden in whom it is felt there is a high chance of achieving lung expansion and space obliteration ensuring the maximal benefit is realized.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (David Waller and Annabel Sharkey) for the series “Mesothelioma Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.05.05). The series “Mesothelioma Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Butchart EG. Contemporary management of malignant pleural mesothelioma. Oncologist 1999;4:488-500. [PubMed]
- Craighead JE. Epidemiology of mesothelioma and historical background. Recent Results Cancer Res 2011;189:13-25. [Crossref] [PubMed]
- Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol 2009;39:576-88. [Crossref] [PubMed]
- Patel SC, Dowell JE. Modern management of malignant pleural mesothelioma. Lung Cancer (Auckl) 2016;7:63-72. [PubMed]
- Peto J, Decarli A, La Vecchia C, et al. The European mesothelioma epidemic. Br J Cancer 1999;79:666-72. [Crossref] [PubMed]
- Muers MF, Stephens RJ, Fisher P, et al. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial. Lancet 2008;371:1685-94. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- van Meerbeeck JP, Gaafar R, Manegold C, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 2005;23:6881-9. [Crossref] [PubMed]
- Gelvez-Zapata SM, Gaffney D, Scarci M, et al. What is the survival after surgery for localized malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg 2013;16:533-7. [Crossref] [PubMed]
- Suda T, Kitamura Y, Hasegawa S, et al. Video-assisted thoracoscopic extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2007;134:1088-9. [Crossref] [PubMed]
- Demmy TL. Video-assisted thoracoscopic extrapleural pneumonectomy for malignant pleural mesothelioma. Ann Cardiothorac Surg 2012;1:533. [PubMed]
- Lang-Lazdunski L. Surgery for malignant pleural mesothelioma: why, when and what? Lung Cancer 2014;84:103-9. [Crossref] [PubMed]
- Valsecchi A, Arondi S, Marchetti G. Medical thoracoscopy: Analysis on diagnostic yield through 30 years of experience. Ann Thorac Med 2016;11:177-82. [Crossref] [PubMed]
- Husain AN, Colby T, Ordonez N, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2013;137:647-67. [Crossref] [PubMed]
- Fysh ET, Tan SK, Read CA, et al. Pleurodesis outcome in malignant pleural mesothelioma. Thorax 2013;68:594-6. [Crossref] [PubMed]
- Rena O, Boldorini R, Papalia E, et al. Persistent lung expansion after pleural talc poudrage in non-surgically resected malignant pleural mesothelioma. Ann Thorac Surg 2015;99:1177-83. [Crossref] [PubMed]
- Barbetakis N, Asteriou C, Papadopoulou F, et al. Early and late morbidity and mortality and life expectancy following thoracoscopic talc insufflation for control of malignant pleural effusions: a review of 400 cases. J Cardiothorac Surg 2010;5:27. [Crossref] [PubMed]
- Bielsa S, Hernandez P, Rodriguez-Panadero F, et al. Tumor type influences the effectiveness of pleurodesis in malignant effusions. Lung 2011;189:151-5. [Crossref] [PubMed]
- Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of pleurodesis. Respiration 2012;83:91-8. [Crossref] [PubMed]
- Srivastava V, Dunning J, Au J. Does video-assisted thoracoscopic decortication in advanced malignant mesothelioma improve prognosis? Interact Cardiovasc Thorac Surg 2009;8:454-6. [Crossref] [PubMed]
- Halstead JC, Lim E, Venkateswaran RM, et al. Improved survival with VATS pleurectomy-decortication in advanced malignant mesothelioma. Eur J Surg Oncol 2005;31:314-20. [Crossref] [PubMed]
- Greengard O, Head JF, Chahinian AP, et al. Enzyme pathology of human mesotheliomas. J Natl Cancer Inst 1987;78:617-22. [PubMed]
- Nakas A, Martin Ucar AE, Edwards JG, et al. The role of video assisted thoracoscopic pleurectomy/decortication in the therapeutic management of malignant pleural mesothelioma. Eur J Cardiothorac Surg 2008;33:83-8. [Crossref] [PubMed]
- Rintoul RC, Ritchie AJ, Edwards JG, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet 2014;384:1118-27. [Crossref] [PubMed]
- Hoang CD. Surgical controversies in mesothelioma: MesoVATS addresses the role of surgical debulking. Transl Lung Cancer Res 2016;5:82-4. [PubMed]
Cite this article as: Ali JM, Aresu G, Peryt A, Coonar AS. Video-assisted thoracoscopic surgery in the management of mesothelioma. Shanghai Chest 2018;2:46.


