
Advances in bronchoscopic management of refractory asthma
Introduction
Characteristic features of asthma include chronic inflammation of the airways, airway wall edema, bronchial hyper-responsiveness, and airway remodeling (1-5). Increased smooth muscle mass in the airway wall is one feature of airway remodeling in asthma. Contraction of these smooth muscles is a primary cause of airway constriction during asthma exacerbations. Despite the introduction of novel targeted pharmacotherapy, approximately 15–20% of patients continue to suffer from severe persistent asthma. They remain symptomatic despite treatment with current state-of-the-art medications (6,7).
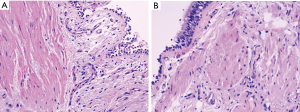
Increased airway smooth muscle mass in asthmatics contributes to bronchospasm and reduced airflow with exposure to allergens (4,5). It has been postulated that a physical reduction of smooth muscle in the airway walls of patients with moderate to severe persistent asthma would reduce the intensity of bronchoconstriction (Figure 1). In turn, this would result in fewer symptoms, improved lung function, and decreased frequency of asthma exacerbations (8).
The current treatment regimen for asthma revolves around the use of inhaled and oral medications. Some intravenous medications, such as omalizumab, a monoclonal antibody targeting IgE, are also available but designed for a small subset of the asthma population. Bronchial thermoplasty (BT) is currently not part of the standard asthma management algorithm. Barriers to widespread adoption include a lack of equipment in some areas and physician training. As more long-term data become available, however, incorporation of this novel technology into guidelines for refractory asthma will hopefully occur.
Supporting data
Studies including patients with varying degrees of asthma severity have repeatedly demonstrated the safety and effectiveness of BT. One of the initial studies in patients with mild to moderate asthma showed improved peak flow rates, increased symptom-free days 3 months post-procedure, and decreased bronchial hyper-responsiveness at 3 and 12 months post-procedure (9). The long-term safety of BT is supported by the absence of qualitative changes in the lungs by CT imaging, lack of decline in FEV1, and the low rate of clinical complications (10).
The Asthma Intervention Research (AIR) trial was a multi-country randomized controlled trial (RCT) involving 11 centers designed to assess the effectiveness and safety of BT in subjects with moderate to severe persistent asthma. One hundred and twelve patients who were already on an inhaled corticosteroid (ICS) and a long-acting beta agonist (LABA) were divided into a BT group and a control group. Patients treated with BT reported significantly fewer exacerbations at 12 months (11). In the BT group, 0.18±0.31 mild exacerbations occurred per subject per week compared to 0.35±0.32 at baseline. The incidence of severe exacerbation in the BT group was also reduced at 0.01±0.08 per subject per week versus 0.07±0.18 at baseline. Results at 3 and 12 months were comparable (P=0.03). At the 1 year mark, the BT group reported the following benefits:
- Eighty-six more symptom-free days (P=0.05);
- Four hundred fewer puffs of rescue medication (P=0.04);
- Ten fewer asthma attacks (mild exacerbations) per year;
- Significant improvement in asthma control (P=0.003) and quality of life (QoL) (P=0.001).
The most common respiratory-related adverse events during the treatment period were dyspnea, wheeze, cough, and chest discomfort. The majority of these adverse events occurred within 1 day of the procedure and resolved an average of 7 days after onset.
The Research in Severe Asthma (RISA) trial was a multi-country RCT in eight centers designed to evaluate the safety and effectiveness of BT in severe persistent asthmatics (9). A total of 32 patients who were already on maximum dose ICS and LABA and up to 30 mg of oral corticosteroids were divided into two groups: BT versus maximal medical therapy. The BT group showed significantly better results after 1-year in the following categories:
- QoL (1.1 increase in asthma QoL score on a scale of 1 to 7 with higher numbers indicating improved QoL) (12);
- Asthma control (0.9 decrease in asthma control questionnaire score on a scale of 0 to 6 with lower numbers indicating improved asthma control) (12);
- Use of rescue medications (25 fewer puffs per week or 6.5 canisters per year);
- FEV1 percent predicted (15.8% improvement in lung function with 12% commonly considered to represent a clinically meaningful change).
Not unexpectedly in this group of severe asthmatics, there was a temporary increase in the severity of respiratory adverse events shortly after BT administration compared to controls. Five hospital admissions occurred within 1 week following a total of 42 BT procedures.
Most recently, the multi-center, RCT, double-blind, sham-controlled Asthma Intervention Research 2 (AIR2) trial was conducted with severe persistent asthmatics. These patients remained symptomatic despite ≥1,000 µg per day of inhaled beclomethasone or equivalent and the maximum dose of a LABA (13).
The study included a total of 297 patients (196 in the BT group and 101 in the sham group) and was conducted in 30 centers from 6 countries. The primary effectiveness endpoint was the difference between study groups in the change in Asthma Quality of Life Questionnaire (AQLQ) score. Pertinent findings for BT treated subjects included:
- Persistence of effect to at least 1 year;
- 84% reduction in emergency department visits for respiratory symptoms;
- Improved asthma QoL from baseline (BT group 1.35±1.10 versus 1.16±1.23 in control group);
- No subject withdrew from the study due to worsening of asthma;
- No deterioration in FEV1 over time;
- 32% reduction in severe exacerbations (0.48±0.067 exacerbations per subject in the BT group versus 0.7±0.1222 in control group);
- 36% reduction in asthma-related adverse events;
- 66% reduction in days lost from work/school/other daily activities due to asthma (1.315±0.361 days per subject in the BT group versus 3.915±1.553 in the control group).
Equipment
The Alair™ Bronchial Thermoplasty System (BTS) is comprised of a radiofrequency (RF) catheter and controller. The RF catheter is inserted through the working channel of a flexible bronchoscope. A foot pedal is used to deliver RF electrical energy after the catheter is extended into the airway and the electrode array at its tip is expanded to contact the airway walls. The airway wall tissue resistance converts the RF energy from the electrode into thermal energy. The controller delivers a measured amount of energy according to preset treatment parameters for a short duration to reduce the risk of unintentional damage to the surrounding structures. A fail-safe mechanism exists if any of the following parameters exceed a pre-programmed limit: length of energy delivered, joules of energy administered, and tissue temperature (14).
Procedural methodology
Three BT sessions at 3-week intervals are recommended. The gap between sessions decreases the likelihood of provoking an asthma exacerbation. The right lower lobe, left lower lobe, and bilateral upper lobes are treated in the first, second, and third sessions, respectively (15). The right middle lobe is spared to reduce the theoretical risk of middle lobe syndrome (8,16). BT is an outpatient procedure and may be performed under moderate sedation or general anesthesia. General anesthesia is preferred in our practice to minimize patient movement given the precise bronchoscopic control required for thorough application of energy in the distal airways.
A flexible bronchoscope with a ≥2.0 mm working channel is recommended for the RF catheter. Our practice is to use a bronchoscope with a 2.0 mm working channel. While a larger bronchoscope can certainly accommodate the RF catheter, its size will generally preclude access to subsegmental and more distal airways.
The RF catheter is inserted into the distal airways while maintaining direct visualization of the black markings (spaced at 5 mm intervals) on the catheter. The catheter will fit into airways ≥3.0 mm. After successful application of energy, the catheter is retracted 5 mm, and the procedure is repeated until the entire airway is covered. As the catheter is retracted into larger airways, the electrode wires are expanded so that all four limbs make contact with airway wall. The appropriate connection of the electrodes is visually confirmed, if possible, after which the foot pedal is depressed and then released.
The electrode array position should be referenced to anatomical landmarks because of the potential for relative motion among the bronchoscope, catheter, and airways. A bronchial map is utilized to ensure that the entire lobe(s) is covered. A methodical approach will ensure no airways are missed or inappropriately re-treated.
The ultimate success of BT depends on various factors including appropriate patient selection, adequate patient preparation, proper and thorough application of energy to all suitable airways, and post-procedure care (17).
Conclusions
BT has been approved by the US Food and Drug Administration (FDA) since April 2010 and is now available in select centers. It has revolutionized treatment for severe persistent asthmatics who remain suboptimally controlled despite maximal medical therapy. Numerous well-designed studies have repeatedly demonstrated the safety and effectiveness of this modality. For a select patient population, it represents a desperately needed breakthrough. As high-quality data continue to be generated and long-term effects are observed, the role of BT will undoubtedly gain further traction and support in treatment algorithms. Patient and physician education of this therapeutic option is paramount.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.06.01). DKH serves as an unpaid editorial board member of Shanghai Chest from Jan 2018 to Dec 2019 and has consulted and lectured for Boston Scientific, has done contracted research for Boston Scientific, and has received an unrestricted education grant from Boston Scientific. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Asthma Education and Prevention Program. (2007) Expert Panel Report III: Guidelines for the diagnosis and management of asthma. Available online: https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf
- Laitinen LA, Heino M, Laitinen A, et al. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis 1985;131:599-606. [PubMed]
- Sobonya RE. Quantitative structural alterations in long-standing allergic asthma. Am Rev Respir Dis 1984;130:289-92. [PubMed]
- Bergeron C, Boulet LP. Structural changes in airway diseases: characteristics, mechanisms, consequences, and pharmacologic modulation. Chest 2006;129:1068-87. [Crossref] [PubMed]
- Carroll N, Elliot J, Morton A, et al. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis 1993;147:405-10. [Crossref] [PubMed]
- Center for Disease Control. National Health Interview Survey, National Center for Health Statistics, CDC. Available online: http://www.cdc.gov/asthma/nhis/06/table3-1.htm
- Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s severe asthma research program. J Allergy Clin Immunol 2007;119:405-13. [Crossref] [PubMed]
- Cox PG, Miller J, Mitzner W, et al. Radiofrequency ablation of airway smooth muscle for sustained treatment of asthma: preliminary investigations. Eur Respir J 2004;24:659-63. [PubMed]
- Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 2007;176:1185-91. [Crossref] [PubMed]
- Cox G, Laviolette M, Rubin A, et al. Long term safety of bronchial thermoplasty (BT): 3 year data from multiple studies. Am J Respir Crit Care 2009;179:A2780.
- Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007;356:1327-37. [Crossref] [PubMed]
- Thomson NC, Rubin AS, Niven RM, et al. Long-term (5 year) safety of bronchial thermoplasty: asthma intervention research (AIR) trial. BMC Pulm Med 2011;11:8. [Crossref] [PubMed]
- Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Resp Crit Care Med 2010;181:116-24. [Crossref] [PubMed]
- Danek CJ, Lombard CM, Dungworth DL, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol 1985;2004:1946-53. [PubMed]
- Cox G, Miller JD, McWilliams A, et al. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 2006;173:965-9. [Crossref] [PubMed]
- Gudmundsson G, Gross TJ. Middle lobe syndrome. Am Fam Physician 1996;53:2547-50. [PubMed]
- Mayse M, Laviolette M, Rubin A, et al. Clinical pearls for bronchial thermoplasty. J Bronchol 2007;14:115-23. [Crossref]
Cite this article as: Ghori UK, Kurman JS, Hogarth DK. Advances in bronchoscopic management of refractory asthma. Shanghai Chest 2018;2:47.


