
Advances in mesothelioma imaging and implications for surgical management
Introduction
Malignant pleural mesothelioma (MPM) is a locally invasive, asbestos-related cancer (1,2) that arises from the pleural surfaces, which encapsulate the lung and thoracic cavity. Surgery is most frequently performed in MPM during diagnostic work-up, but may also be offered in selected patients as part of a radical multi-modality treatment strategy, by extended pleurectomy/decortication (EP/D), or in historical series by extra-pleural pneumonectomy (EPP). Occasionally, palliative surgery may be offered in patients with symptomatic pleural effusion and/or associated trapped lung. The evidence associated with surgery in these settings has been reviewed elsewhere in this issue. This article will focus on the many recent developments in MPM imaging, which have the potential to enhance pre-, intra- and post-operative decision making. We have structured this article around the key clinical scenarios in which MPM surgery may be offered but have not included any data regarding use of the Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST criteria in MPM, since these are not routinely used to assess surgical response. However, MPM surgery is likely to remain one component of multi-modality regimens and an understanding of this topic is essential. Readers are therefore directed to relevant publications on this topic (3-5).
Imaging for diagnostic surgery
MPM presents in an undifferentiated fashion, most frequently with breathlessness +/− pain, associated with a unilateral pleural effusion +/− mass. The low sensitivity of fluid cytology, which is frequently the first invasive test performed, for MPM (effectively zero in many non-specialist cytology centres (6,7) places great importance on the perceived performance of the imaging conducted at first presentation. These results will often be used to justify surveillance vs. immediate histological sampling, ideally by video-assisted thoracoscopic surgery (VATS) or local anaesthetic thoracoscopy (LAT), which both offer >90% MPM sensitivity (8).
Chest radiograph (CXR)
The CXR will typically reveal a pleural effusion +/− loss of hemi-thoracic volume, nodular pleural thickening, fissural thickening or a pleural mass, but these are insensitive and non-specific features (9,10). Right-sided abnormalities are more common and bilateral disease is exceptionally rare (11). Calcified (or non-calcified) pleural plaques are consistent with prior asbestos exposure, but are not specific markers for MPM (12,13).
Thoracic ultrasound (TUS)
TUS assessment should be part of the standard work-up for all patients with suspected pleural malignancy, including MPM. Advantages including speed, ease of use and mobility. Use of a convex, low-frequency transducer probe (e.g., 3.5 MHz) allows visualization and estimation of pleural effusion volume, identification of tumour nodules (14,15) and selection of a safe site for fluid aspiration (16). A higher frequency, linear probe (5 or 7.5 MHz) allows more detailed assessment of the chest wall and parietal pleura. Sonographic evidence of nodular pleural thickening, pleural thickening >1 cm +/− diaphragmatic nodules are highly specific (95–100%) markers of pleural malignancy in general, but offer poor sensitivity (40%) (17). Therefore, a bland TUS should not preclude further investigation. TUS findings can also be helpful in determining the most appropriate biopsy strategy, particularly in selecting patients for VATS, in preference to LAT. While LAT is suitable for most patients and can frequently be performed locally without onward referral, a highly loculated pleural space may be better managed using VATS. Recent advances in ultrasound application include use of M-mode and Speckle Tracking analyses to non-invasively predict non-expansile lung (NEL) (18). Given the evolving importance of NEL in MPM (see ‘Imaging prior to Palliative Surgery’ section), these may be worth integrated into clinical practice, if validated in larger studies.
Computed tomography (CT)
Technical considerations
Multi-slice CT facilitates detailed examination of any body part and isotropic multi-planar image reformatting. Optimal CT assessment requires volume imaging, 60–90 seconds after iodinated contrast injection (19). CT pulmonary angiography is insensitive to MPM (27% in a recent study) (20) and is of very limited value. The imaged volume must include the thorax and abdomen, including the inferior costophrenic sulci.
Typical morphological CT features
Pleural effusion and pleural thickening are common but non-specific CT features. Pleural plaques are visible on CT in 20% (21) to 43% (22) of patients with MPM, but have been more frequently reported in cohorts of benign pleural disease (23). No CT feature reliably differentiates MPM from metastatic pleural malignancy, which in clinical practice is often the primary question, although circumferential and mediastinal pleural thickening are more common in MPM (24).
Real-life diagnostic performance
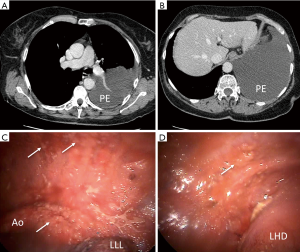
Previous small studies suggested that CT was very accurate for detection of pleural malignancies, including MPM, with morphological abnormalities (e.g., pleural enhancement, nodular or mediastinal pleural thickening) being associated with high sensitivity (96%) and specificity (80%) (23). However, cross-sectional imaging is uniquely challenging in MPM. The disease is distributed heterogeneously over a large surface area and adopts a sessile (flat) configuration in early stage disease. In our experience, early stage MPM is frequently CT-occult but easily visualized at LAT, see Figure 1 for an example. This is corroborated by two recent studies regarding the real-life performance of CT in this setting. Hallifax et al. reported only 68% sensitivity [negative predictive value (NPV 65%)] (25) in 370 patients referred for LAT, while Tsim et al. reported a 58% sensitivity (NPV 54%) in 315 patients (20) recruited to the DIAPHRAGM study (ISRCTN 10079972), at first presentation of MPM (26). This relative insensitivity of CT may well be an important factor in the frequent diagnostic delays experienced by MPM patients (27). An efficient diagnostic pathway therefore requires recognition that the only CT (20,25) [or TUS (17)] abnormality in MPM may be a new, bland pleural effusion. CT may help selection of the most appropriate biopsy procedure, e.g., tumour nodules may be most amenable to image-guided biopsy, but fluid loculation is poorly visualized on CT and selection of LAT vs. VATS should incorporate other data.
Perfusion CT
Perfusion CT involves sequential high-resolution image acquisition after injection of iodinated contrast. This allows estimation of the tumour micro-vasculature, based on blood flow, volume and capillary permeability (28), which has potentially significant clinical application given the emerging importance of anti-angiogenic therapies, including bevacizumab (29) and nintedanib (30). A recently reported prospective pilot study described a potential treatment-specific fall in tumour blood volume and perfusion in 8 MPM patients receiving various therapies (31,32), but these data require further validation. CT perfusion is limited in this regard, since it involves high radiation exposure. Evolving, low-dose CT techniques, incorporating iterative reconstruction (33), projection view sharing (34) and reductions in tube current-time product and voltage may abrogate some of these concerns but at present, they limit applications of the technique.
Positron emission tomography (PET) and PET-CT
Technical considerations
PET exploits increased uptake of radioactive metabolic tracers [e.g., 18fluoro-deoxy-glucose (FDG)] by cancer cells to generate relatively selective imaging. Integrated PET-CT combines metabolic PET data with CT, overcoming the low spatial resolution of PET. Patients are typically fasted for 4–6 hours before injection of 3.5–5.2 MBq/kg of 18FDG, 60–120 minutes before scanning. Maximal tracer uptake can then be recorded within user-defined regions of interest, generating end-points including peak standardized uptake value (SUVmax) and total glycolytic volume (TGV), which integrates SUV and estimated pleural tumour volume. SUV values are influenced by patient characteristics, e.g., blood glucose and technical factors, e.g., scanner and parameters chosen.
Diagnostic performance
SUVmax is typically higher in MPM [reported mean SUVmax 6.5 (3.4)] than in benign pleural disease [reported mean SUVmax 0.8 (0.60) (35)], but PET-CT has a limited role in primary diagnostics. This reflects limited availability, but also contradictory meta-analyses [sensitivity 95%, specificity 82% (36) vs. 81% sensitivity, 74% specificity (37)], which highlight that false negatives may occur in early stage MPM, because, similar to small (<8 mm) bronchial neoplasms (38), sensitivity is reduced in small volume pleural tumours. Additionally, false positives may result from inflammatory/infectious pleurites, such as rheumatoid pleuritis, tuberculosis, and prior talc pleurodesis (35-37).
Biopsy planning
Theoretically, PET-CT can be used to select the best site for biopsy and is used in some centres for this purpose. The multi-centre, randomised TARGET trial (ISRCTN 14024829) is currently recruiting in the UK, to determine whether this improves diagnostic accuracy, relative to operator-selected biopsy using standard CT images.
Magnetic resonance imaging (MRI)
Technical considerations
MRI utilizes electromagnets to generate a magnetic field, which can be harnessed to polarize (or excite) tissue protons and to detect energy released during their subsequent relaxation. Modern systems generate field strengths up to 7-Tesla (T), but most clinical systems operate at 1.5–3-T. MRI is ideally suited to MPM, because of high spatial resolution and the high natural contrast provided by adjacent, proton (water)-rich, pleural effusion. Pleural fluid demonstrates low (dark) signal on T1-weighted images due to the slower T1 relaxation of free water relative to adjacent tissues (e.g., fat). On T2-weighted imaging, free water within a pleural effusion is high (bright) in signal, optimizing detection of septa. This is particularly helpful in the selection of cases for VATS over LAT. Paramagnetic gadolinium-based contrast agents can be used to enhance contrast between tissues, and are limited only by a significant renal impairment (39). In many centres, MRI remains an ancillary diagnostic or staging tool due to increased scan times and lower availability, relative to CT.
Typical morphological features
The morphological features of MPM on MRI are similar to those on CT. However, previous studies demonstrate superior sensitivity (91–100%) and specificity (73–80%) (23,40) for MRI relative to CT. However, the real-life analyses of CT performance recently reported (20,25) have not been possible for MRI, reflecting its less frequent clinical use.
Diffusion-weighted MRI (DWI-MRI)
In 2010, Gill et al. first described DWI-MRI in MPM, reporting that the apparent diffusion coefficient (ADC), a measure of the relative diffusion of water molecules within tissues, was reduced in the pleura of patients with MPM, relative to those with benign disease (41). In addition, epithelioid MPM was associated with higher ADC values than sarcomatoid MPM (41). Coolen et al. subsequently confirmed this and reported 71% sensitivity and 100% specificity, based on an ADC threshold of 1.52×10−3 mm2/s (42). The same authors subsequently reported a subjective correlate termed ‘pleural pointillism’, which describes inhomogeneous pleural hyperintensity on high b-values DW images (1,000 s/mm2). In a larger study (n=109, 57 of whom had MPM), this was associated with 93% sensitivity and 79% specificity for pleural malignancy (43). Pointillism, named after the post-impressionistic painting technique it resembles when present, has the advantage of simplicity of reporting over computation of ADC values, but the disadvantage of greater subjectivity. Coolen et al. reported a κ of only 0.53, which was lower than subjective morphology in the same study (e.g., mediastinal thickening κ 0.71) (43). Moreover, the ‘added value’ of DWI-MRI, needs further clarification, given the additional time required for acquisition and reporting. MPM staging was not reported by Coolen et al, but 67% patients had ‘shrinking lung’ (43), which is generally a feature of late stage disease where morphology alone performs well. Nevertheless, pointillism is quick to report and DW-MRI may prove valuable, subject to validation in other centres.
Perfusion MRI
Unlike perfusion CT, perfusion MRI is not limited by high radiation exposure, and more promising applications exist. These include dynamic contrast enhanced MRI (DCE-MRI) and early contrast enhancement MRI (ECE-MRI).
DCE-MRI
DCE-MRI is a direct correlate of CT perfusion and allows computation of various pharmacokinetic parameters. Giesel et al. reported kinetic values that correlated with tumour vascularity and vascular permeability and were predictive of response to anti-angiogenic therapy in 19 patients (44,45). Coolen et al. subsequently found that use of DCE-MRI in cases with indeterminate DWI-MRI increased sensitivity (71% to 93%) at the cost of slightly reduced specificity (100% to 94%) (42). However, a major drawback of DCE-MRI is that it requires a visible tumour mass for deployment, limiting its genuine ‘added value’ over standard diagnostic morphology.
ECE-MRI
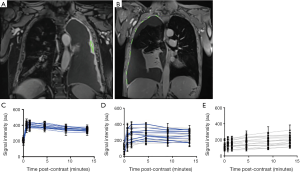
ECE-MRI is a recently reported technique that, unlike DCE-MRI can be applied in patients with minimal pleural thickening (32). In a recent pilot study, Tsim et al. acquired coronal 3D spoiled gradient echo sequences during breath holding before and repeatedly after gadolinium contrast. Peak signal intensity was measured in up to 15 user-defined regions of interest; including areas of bland pleural thickening if no tumour was visible (see Figure 2 for examples). Peak enhancement occurred at or before 4.5minutes (labelled by the authors as ECE), and correlated with tumour micro-vessel density (MVD) (32) and adverse survival. Although labelled ECE, this time-point is later than peak enhancement after iodinated contrast [60–90 seconds (19)] but is concordant with a study recently reported by Armato et al., in which MRI peak enhancement occurred after 280 seconds (32). This delayed enhancement interval may reflect factors other than the microvasculature, including delayed clearance of gadolinium (an extra-cellular agent) from peri-tumoral stroma, which is particularly prevalent in MPM (46). In contrast to pleural pointillism, inter-observer agreement for MRI-ECE (κ 0.784) exceeded that for subjective CT (κ 0.65) and MRI morphology (κ 0.593), possibly reflecting the semi-objective definition of ECE. If validated in larger studies, MRI-ECE might genuinely add ‘disease detection’ value over traditional morphology, which could increase the proportion of patients eligible for potentially radical surgery.
Imaging prior to radical surgery for MPM
The mesothelioma multi-disciplinary team (MDT)
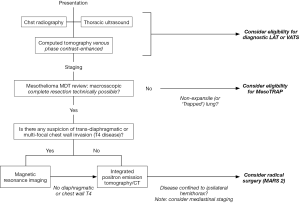
Radiological assessment prior to radical surgery should be undertaken by a dedicated Mesothelioma MDT. MDT working improves diagnostic performance and is associated with recruitment to trials (47). The value of the MDT in concentrating expertise cannot be over-stated, given the low incidence of MPM and the challenges involved in MPM diagnostics and staging (48-51). Successful assessment prior to radical surgery requires an understanding of (I) the anatomy and the definitions of potential resectability; (II) the strengths and weaknesses of each imaging modality; and (III) the likely impact on the patient of under- or over-staging. An integrated approach to imaging prior to radical surgery is summarized in Figure 3.
Managing uncertainty
The aim of potentially radical surgery in MPM is macroscopic complete resection (MCR) and failure to achieve this adversely affects prognosis (52,53). The extent of apparent pre-operative involvement should therefore always be weighted, realistically, against the surgical expectation to achieve MCR. In our opinion, multiple or large areas of doubt regarding resectability should contraindicate surgery in most cases. A commonly encountered area of uncertainty is the definition of what is anatomically ‘resectable’. While there is broad consensus that T4 or N2 or M1 disease should be considered unresectable, several factors may influence the decision to offer surgery in borderline cases (e.g., T3 possibly T4, possible mediastinal N1 disease) and these are discussed in detail below. These should balance the risks of pre-operative over-staging and exclusion from potentially beneficial surgery vs. under-staging and overly aggressive, futile surgery. However, previous studies frequently report up-staging of patients at surgery. In 2012, Rusch et al. reported upstaging in up to 80% of 1,056 MPM patients treated surgically for pre-operatively (or clinically) staged stage I or II disease, and 23% of patients with pre-operative stage III disease (54).
Identification of technically unresectable T4 disease
Since T3 is defined as locally advanced, potentially resectable tumour and T4 as technically unresectable tumour, differentiation between these requires previous experience. Consequently, centre- and volume-dependent factors become important in making high quality pre-surgical staging decisions. CT allows a reasonable assessment of lung involvement (i.e., T1 vs. T2 disease), a greater extent of which may be acceptable in cases of intended lung removal (EPP). However, MRI is superior to CT in detecting invasion of the chest wall, diaphragm and bony structures, which will constitute at least T3 disease (55,56). Stewart et al. performed contrast-enhanced 1.5-T MRI on 69 patients with apparently resectable MPM following contrast-enhanced CT scanning, and found CT-occult, unresectable (T4) disease in 17/76 (22%) patients (57). PET-CT is relatively insensitive to extra-pleural invasion, as shown by a previous report of 67% sensitivity for T4 disease (58). Therefore, in cases where diagnostic CT imaging demonstrates T3 (and therefore potential T4) disease (e.g., a single focus of chest wall invasion) or the patient has symptoms suggestive of multi-focal chest wall invasion (e.g., severe chest pain) regardless of CT T-stage, it is our practice to perform contrast-enhanced MRI if radical surgery is being considered.
Tumour volumetry
In lung cancer, recent data have demonstrated the powerful prognostic impact of small increases in primary tumour size, resulting in adoption of 1 cm increments in T-stage descriptors in the updated staging system (59). In MPM, the technical challenges involved measurements of this precision are greater due to the tumour’s rind-like growth pattern and the complex shape of the pleural cavity. Nevertheless, Nowak et al. recently reported that unidimensional measures of maximum pleural tumour thickness were consistently associated with decreasing survival, node positivity and overall stage in the updated MPM staging database (48). However, unidimensional measurements are limited by inter-observer variability, with Armato et al. reporting up to 30% variance between reporters (60). Computer-aided analysis can improve consistency but this remains high in minimally-measurable (<7.5 mm) lesions (61,62) and cannot overcome obvious concerns regarding poor representation of the overall disease. Volumetry is the logical solution to this but is complicated by further technical challenges, which are gradually being addressed. Either CT, MRI or Integrated PET/CT can be used, but the higher contrast resolution afforded by MRI relative to CT (55,63-65), particularly in resolving tumour from adjacent effusion, renders it a potentially more powerful, but less studied volumetric tool. Using CT, Pass (66) and Gill (67) both reported that above-median MPM tumour volumes (>100 cm3 in 48 patients, >500 cm3 in 88 patients) were associated with adverse survival in single centre analyses. Kircheva et al. also reported that resected tumour volume, measured by water displacement, was a better predictor of survival than T stage, based on current clinical descriptors (68). Plathow et al. reported the only published analysis of MRI volumetry in MPM but did not relate volumetric results to survival. However, MRI volumetry did out-perform CT in determining therapy response according to modified RECIST criteria (69). The larger, multi-centre volumetric CT study (n=164, 129 of whom were analysed) was recently able to define 3 prognostic groups based on cut-points of 91.2, 245.3 and 511.3 cm3, with associated median survival times of 37, 18 and 8 months, respectively (70). However, this study also identified significant inter-observer variability (71). More evolved computational techniques, e.g., the random walk-based method recently reported by Chen et al. (72), are required to address this, and to reduce the time required to report volumetric studies. Deployment of these techniques in larger cohorts, using agreed software is required for validation of volume-based T descriptors that might augment or replace the current descriptive definitions. Use of MRI might also reduce inter-observer variation in comparison to CT (73), but issues related to availability and reporter familiarity need to be overcome. Addition of volumetric metabolic data may also help to select patients for radical surgery, given the powerful prognostic impact of volumetric PET-CT, recently reported by Nowak et al. (74).
Intra-operative identification of residual disease
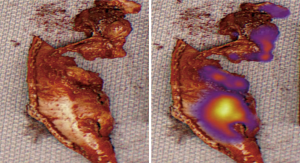
It has long been acknowledged that it may be difficult to identify small volumes of residual tumour intra-operatively (75) and thus achieve MCR. Keating et al. recently reported results of a small pilot study design to augment this assessment, using intra-operative detection of indocyanine green (ICG), a near-infrared (NIR) optical contrast agent that localizes to areas of tumour via enhanced permeability and retention. ICG (5 mg/kg) was injected intravenously in 8 patients 24 hours prior to surgery. After what was thought to be MCR, the wound bed was imaged intra-operatively using a NIR device, revealing NIR fluorescent residual disease in all 8 patients, from whom 1 to 4 additional areas of tumour were resected (mean 1.8), and confirmed histologically (32) (see Figure 4). This potentially important data has yet to be published in full, but warrants further study.
Identification of nodal involvement
Methodological and practical issues
The sub-classification of hilar (N1) and mediastinal (N2) lymph nodes used in lung cancer is not valid in MPM, in which setting malignant lymph may drain directly into the mediastinum (76,77). This is reflected in the 8th edition for TNM staging manual for MPM (49), in which mediastinal nodes have been re-classified as N1. No randomised data exist to inform decisions regarding the appropriateness of EP/D in patients with mediastinal node involvement, which may frequently be technically resectable, albeit extra-pleural and associated with considerable survival disadvantage (49). The nodal staging literature is also complicated in MPM by a tendency to compare one imaging modality with another, with less frequent use of gold-standard histological confirmation than is the case with T-staging, and considerable variation in the extent of nodal dissection during surgery, depending on operator, institution and procedure (49). The latter results in considerable variation in the reported incidence of nodal metastases and contributes to the limited histological reference standards for imaging studies. In addition, the location and distribution of abnormal nodes in MPM [e.g., peri-diaphragmatic (PD), peri-cardiac (PC), see Figure 5 for an example] may preclude comprehensive staging using minimally-invasive endoscopic techniques, such as endobronchial ultrasound (EBUS).
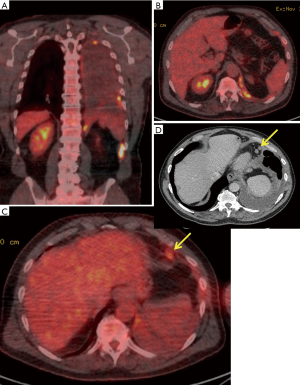
Imaging of nodal metastases
CT is of limited value in assessment of the mediastinum in MPM. Pathological nodes are frequently missed or over-called (previous studies report AUC values of <0.5) (56) and nodal size on CT and pathological status have shown no correlation in previous studies (56). Isolated PET should also not be used since it offers low sensitivity (11%) (78). In a previous comparative study, integrated PET-CT delivered the highest accuracy for nodal metastases (see Figure 5 for examples), relative to isolated PET, CT and MRI (79). In this study, Plathow et al. reported 100% sensitivity and 100% specificity for N1 nodal metastases using PET-CT in 54 patients, 52 of whom had surgical verification of stage. However, neither the nature of the surgery nor the extent of the nodal dissection performed was reported (79). These perfect results have not been replicated by other groups. Sørensen et al. reported 50% sensitivity, 75% specificity, 50% positive predictive value (PPV) and 50% NPV for integrated PET-CT prior to EPP in 24 patients. Erasmus et al. reported 38% sensitivity, 78% specificity, 60% PPV and 58% NPV using the same technique in a similar cohort. The case for investigation beyond CT is well made by Sørensen et al, who reported that PET-CT prevented futile surgery in 12/42 (29%) patients, due to distant metastases or T4 disease (80). Although integrated PET-CT is the most accurate test (79), it should not be relied upon in isolation (58,80). Suspicious sites should be sampled where possible although the extent to which these results should influence surgical decision making is an area of debate.
Current practice
Several centres have recently reported their outcomes following radical surgery (EPP or EP/D), including many patients with mediastinal node involvement. In 2012, Nakas et al. reported that only positive ‘accessible’ [to cervical mediastinoscopy (CM)] mediastinal nodes, in stations 3a, 4 and 7, were associated with a survival disadvantage in 212 patients who underwent EPP or EP/D in the UK. Patients with positive, ipsilateral extra-mediastinal or ‘inaccessible’ (to CM) nodes [internal mammary (IM), PC, PD, stations 5 and 6] had a similar survival to patients with N0 disease and prognostic data from EBUS staging of lower mediastinal stations was non-contributory (81). This non-comparative data has led some to offer radical surgery to patients with radiologically abnormal IM, PC, PD or station 5/6 nodes as long as these are technically resectable (Nakas 2012), but ideally after a staging CM to identify superior mediastinal (N1) disease (82,83). In a contradictory US series, mediastinal node involvement had no impact on subsequent survival (68). In the currently recruiting MARS-2 trial an intermediate approach has been adopted. No specific nodal staging beyond CT is mandatory and patients can be allocated to EP/D so long as their disease is technically resectable and confined to one hemi-thorax. Mediastinal (N1) disease is not therefore an absolute exclusion criterion to EP/D in this important trial.
Identification of metastatic disease
Metastatic disease may be more common in MPM than has been traditionally been taught. In a recent, large post-mortem series (n=318) extra-pleural disease was evident in 87.7% of patients and extra-thoracic disease in 55.4% (84). Case reports of unusual metastatic sites (muscle, skin and colon) have also recently been published (85-87). Given its widespread availability and low-cost contrast-enhanced CT should initially be used for metastatic staging. However integrated PET-CT is more sensitive (58,78) and should be considered after CT if radical surgery is contemplated. For equivocal extra-thoracic abnormalities MRI may occasionally be useful, e.g., regarding potential bone lesions.
Imaging prior to palliative surgery
Partial P/D (PP/D) may be helpful in palliating selected patients, particularly those with a gross visceral pleural cortex or a malignant empyema (88). In this setting, pre-operative cross-sectional imaging (by CT +/− MRI) allows some estimation of the relative risks and benefits of the procedure, with large tumour volumes and wider total trapped lung surface decreasing the chances of good technical and symptomatic success. However, the evidence base for palliative surgery is currently limited. In the MesoVATS trial, PP/D demonstrated no survival advantage over simple talc pleurodesis, and was associated with increased complications and increased cost (89). However, patients with NEL were largely excluded and might have the most to gain from this procedure. The Meso-TRAP trial has therefore been initiated to determine the value of PP/D in patients with trapped lung (or NEL) relative to placement of an IPC, which is the standard of care in this setting (90). The study is currently recruiting patients to a randomised feasibility phase, including the following inclusion criterion for: ‘clinically significant trapped lung’. Accurate and consistent radiological detection of this is therefore of fundamental importance, but poses considerable and often underestimated problems.
Radiological definition of ‘trapped’ or NEL
NEL can be simply defined as failure of full lung re-expansion after drainage of pleural fluid, and may result from malignant or reactive visceral pleural thickening, proximal endobronchial obstruction or reduced parenchymal compliance. A thick visceral cortex may be obvious on CT prior to first pleural intervention, but in the absence of this, NEL can be difficult to predict using baseline radiology (CXR, CT or MRI). This is reflected in a significant proportion of patients admitted for an attempt at fluid drainage and talc pleurodesis not receiving talc because of unexpected NEL, including up to 25% of patients recruited recent pleurodesis phase III trials. Emerging ultrasound end-points might enhance NEL prediction prior to fluid drainage, including M-mode and speckle tracking imaging analyses (18) (see TUS section), but NEL can also be challenging to detect after fluid is removed. Serial CXRs may reveal a classic (ex vacuo) pneumothorax, but Martin et al. recently reported considerable disagreement between two reporters regarding the presence of post-drainage NEL. The subjective method currently advocated by the BTS (based on less than 50% re-apposition of the pleural surfaces), was associated with a κ of only 0.68 and 81% sensitivity/87% specificity. During surgical thoracoscopy, NEL can be directly and accurately visualized based on the extent of lung re-inflation during positive pressure ventilation, allowing a judgement to be made regarding IPC vs. talc pleurodesis (91). However, visual assessment performs poorly during LAT, with only 12.5% cases of NEL correctly identified in a recent survey, although this study was small, retrospective and judgements were based on 30 second video-clips only (92). Given the emerging potential importance of NEL in selecting MPM patients for PP/D, better radiographic correlates of this phenomenon are urgently required.
Conclusions
The peculiar biology and unique growth pattern of MPM pose particular imaging challenges. Nevertheless, there have been significant recent developments in diagnostic imaging and radiological staging that may facilitate better surgical decision-making in MPM patients and ultimately better outcomes. However, many require further prospective evaluation in large, multi-national collaborative studies.
Acknowledgments
Funding: KG Blyth is part-funded by a National Health Service Research Scotland Senior Fellowship and acknowledges recent relevant grant funding from the Chief Scientist’s Office (ETM/285), the British Lung Foundation (MPG16-7) and the June Hancock Mesothelioma Research Fund (JH/17/03).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (David Waller and Annabel Sharkey) for the series “Mesothelioma Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.07.01). The series “Mesothelioma Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med 1960;17:260-71. [PubMed]
- McElvenny DM, Darnton AJ, Price MJ, et al. Mesothelioma mortality in Great Britain from 1968 to 2001. Occup Med (Lond) 2005;55:79-87. [Crossref] [PubMed]
- Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 2004;15:257-60. [Crossref] [PubMed]
- Tsao AS, Garland L, Redman M, et al. A practical guide of the Southwest Oncology Group to measure malignant pleural mesothelioma tumors by RECIST and modified RECIST criteria. J Thorac Oncol 2011;6:598-601. [Crossref] [PubMed]
- Cheng L, Tunariu N, Collins DJ, et al. Response evaluation in mesothelioma: Beyond RECIST. Lung Cancer 2015;90:433-41. [Crossref] [PubMed]
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Husain AN, Colby T, Ordonez N, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2013;137:647-67. [Crossref] [PubMed]
- Hooper C, Lee YC, Maskell N, et al. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii4-17. [Crossref] [PubMed]
- Adams VI, Unni KK, Muhm JR, et al. Diffuse malignant mesothelioma of pleura. Diagnosis and survival in 92 cases. Cancer 1986;58:1540-51. [Crossref] [PubMed]
- Salonen O, Kivisaari L, Standertskjöld-Nordenstam CG, et al. Computed tomography of pleural lesions with special reference to the mediastinal pleura. Acta Radiol Diagn (Stockh) 1986;27:527-31. [Crossref] [PubMed]
- Beckett P, Edwards J, Fennell D, et al. Demographics, management and survival of patients with malignant pleural mesothelioma in the National Lung Cancer Audit in England and Wales. Lung Cancer 2015;88:344-8. [Crossref] [PubMed]
- Pairon JC, Laurent F, Rinaldo M, et al. Pleural plaques and the risk of pleural mesothelioma. J Natl Cancer Inst 2013;105:293-301. [Crossref] [PubMed]
- Paris C, Thierry S, Brochard P, et al. Pleural plaques and asbestosis: dose- and time-response relationships based on HRCT data. Eur Respir J 2009;34:72-9. [Crossref] [PubMed]
- Görg C, Restrepo I, Schwerk WB. Sonography of malignant pleural effusion. Eur Radiol 1997;7:1195-8. [Crossref] [PubMed]
- Yang PC, Luh KT, Chang DB, et al. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol 1992;159:29-33. [Crossref] [PubMed]
- Havelock T, Teoh R, Laws D, et al. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii61-76. [Crossref] [PubMed]
- Qureshi NR, Rahman NM, Gleeson FV. Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax 2009;64:139-43. [Crossref] [PubMed]
- Salamonsen MR, Lo AKC, Ng ACT, et al. Novel use of pleural ultrasound can identify malignant entrapped lung prior to effusion drainage. Chest 2014;146:1286-93. [Crossref] [PubMed]
- Raj V, Kirke R, Bankart MJ, et al. Multidetector CT imaging of pleura: comparison of two contrast infusion protocols. Br J Radiol 2011;84:796-9. [Crossref] [PubMed]
- Tsim S, Stobo DB, Alexander L, et al. The diagnostic performance of routinely acquired and reported computed tomography imaging in patients presenting with suspected pleural malignancy. Lung Cancer 2017;103:38-43. [Crossref] [PubMed]
- Kawashima A, Libshitz HI. Malignant pleural mesothelioma: CT manifestations in 50 cases. AJR Am J Roentgenol 1990;155:965-9. [Crossref] [PubMed]
- Seely JM, Nguyen ET, Churg AM, et al. Malignant pleural mesothelioma: computed tomography and correlation with histology. Eur J Radiol 2009;70:485-91. [Crossref] [PubMed]
- Hierholzer J, Luo L, Bittner RC, et al. MRI and CT in the differential diagnosis of pleural disease. Chest 2000;118:604-9. [Crossref] [PubMed]
- Metintas M, Ucgun I, Elbek O, et al. Computed tomography features in malignant pleural mesothelioma and other commonly seen pleural diseases. Eur J Radiol 2002;41:1-9. [Crossref] [PubMed]
- Hallifax RJ, Haris M, Corcoran JP, et al. Role of CT in assessing pleural malignancy prior to thoracoscopy. Thorax 2015;70:192-3. [Crossref] [PubMed]
- Tsim S, Kelly C, Alexander L, et al. Diagnostic and Prognostic Biomarkers in the Rational Assessment of Mesothelioma (DIAPHRAGM) study: protocol of a prospective, multicentre, observational study. BMJ Open 2016;6:e013324 [Crossref] [PubMed]
- National Lung Cancer Audit Report 2014 (Mesothelioma) 2016:1-27.
- Drevs J, Schneider V. The use of vascular biomarkers and imaging studies in the early clinical development of anti-tumour agents targeting angiogenesis. J Intern Med 2006;260:517-29. [Crossref] [PubMed]
- Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14. [Crossref] [PubMed]
- Grosso F, Steele N, Novello S, et al. Nintedanib Plus Pemetrexed/Cisplatin in Patients With Malignant Pleural Mesothelioma: Phase II Results From the Randomized, Placebo-Controlled LUME-Meso Trial. J Clin Oncol 2017;35:3591-600. [Crossref] [PubMed]
- Armato SG III, Labby ZE, Coolen J, et al. Imaging in pleural mesothelioma: A review of the 11th International Conference of the International Mesothelioma Interest Group. Lung Cancer 2013;82:190-6. [Crossref] [PubMed]
- Armato SG 3rd, Blyth KG, Keating JJ, et al. Imaging in pleural mesothelioma: A review of the 13th International Conference of the International Mesothelioma Interest Group. Lung Cancer 2016;101:48-58. [Crossref] [PubMed]
- Geyer LL, Schoepf UJ, Meinel FG, et al. State of the Art: Iterative CT Reconstruction Techniques. Radiology 2015;276:339-57. [Crossref] [PubMed]
- Martin T, Hoffman J, Alger JR, et al. Low-dose CT perfusion with projection view sharing. Med Phys 2018;45:101-13. [Crossref] [PubMed]
- Yildirim H, Metintas M, Entok E, et al. Clinical value of fluorodeoxyglucose-positron emission tomography/computed tomography in differentiation of malignant mesothelioma from asbestos-related benign pleural disease: an observational pilot study. J Thorac Oncol 2009;4:1480-4. [Crossref] [PubMed]
- Treglia G, Sadeghi R, Annunziata S, et al. Diagnostic accuracy of 18F-FDG-PET and PET/CT in the differential diagnosis between malignant and benign pleural lesions: a systematic review and meta-analysis. Acad Radiol 2014;21:11-20. [Crossref] [PubMed]
- Porcel JM, Hernández P, Martínez-Alonso M, et al. Accuracy of fluorodeoxyglucose-PET imaging for differentiating benign from malignant pleural effusions: a meta-analysis. Chest 2015;147:502-12. [Crossref] [PubMed]
- Gould MK, Maclean CC, Kuschner WG, et al. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 2001;285:914-24. [Crossref] [PubMed]
- Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. J Am Soc Nephrol 2006;17:2359-62. [Crossref] [PubMed]
- Falaschi F, Battolla L, Zampa V, et al. Comparison of computerized tomography and magnetic resonance in the assessment of benign and malignant pleural diseases. Radiol Med 1996;92:713-8. [PubMed]
- Gill RR, Umeoka S, Mamata H, et al. Diffusion-weighted MRI of malignant pleural mesothelioma: preliminary assessment of apparent diffusion coefficient in histologic subtypes. AJR Am J Roentgenol 2010;195:W125-30 [Crossref] [PubMed]
- Coolen J, De Keyzer F, Nafteux P, et al. Malignant pleural disease: diagnosis by using diffusion-weighted and dynamic contrast-enhanced MR imaging--initial experience. Radiology 2012;263:884-92. [Crossref] [PubMed]
- Coolen J, De Keyzer F, Nafteux P, et al. Malignant pleural mesothelioma: visual assessment by using pleural pointillism at diffusion-weighted MR imaging. Radiology 2015;274:576-84. [Crossref] [PubMed]
- Giesel FL, Bischoff H, von Tengg-Kobligk H, et al. Dynamic contrast-enhanced MRI of malignant pleural mesothelioma: a feasibility study of noninvasive assessment, therapeutic follow-up, and possible predictor of improved outcome. Chest 2006;129:1570-6. [Crossref] [PubMed]
- Giesel FL, Choyke PL, Mehndiratta A, et al. Pharmacokinetic analysis of malignant pleural mesothelioma-initial results of tumor microcirculation and its correlation to microvessel density (CD-34). Acad Radiol 2008;15:563-70. [Crossref] [PubMed]
- O'Byrne K, Rusch V. Malignant Pleural Mesothelioma. Oxford University Press, USA, 2006:1.
- Tsim S, Dick C, Roberts F, et al. 76 Early experience of a regional mesothelioma MDT in the West of Scotland. Lung Cancer 2014;83:S28-9. [Crossref]
- Nowak AK, Chansky K, Rice DC, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2089-99.
- Rice D, Chansky K, Nowak A, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the N Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2100-11.
- Rusch VW, Chansky K, Kindler HL, et al. The IASLC Mesothelioma Staging Project: Proposals for the M Descriptors and for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Mesothelioma. J Thorac Oncol 2016;11:2112-9. [Crossref] [PubMed]
- Rusch VW, Giroux D. Do we need a revised staging system for malignant pleural mesothelioma? Analysis of the IASLC database. Ann Cardiothorac Surg 2012;1:438-48. [PubMed]
- Bölükbas S, Eberlein M, Fisseler-Eckhoff A, et al. Radical pleurectomy and chemoradiation for malignant pleural mesothelioma: the outcome of incomplete resections. Lung Cancer 2013;81:241-6. [Crossref] [PubMed]
- Rusch V, Baldini EH, Bueno R, et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: meeting summary of the International Mesothelioma Interest Group Congress, September 11-14, 2012, Boston, Mass. J Thorac Cardiovasc Surg 2013;145:909-10. [Crossref] [PubMed]
- Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol 2012;7:1631-9. [Crossref] [PubMed]
- Knuuttila A, Halme M, Kivisaari L, et al. The clinical importance of magnetic resonance imaging versus computed tomography in malignant pleural mesothelioma. Lung Cancer 1998;22:215-25. [Crossref] [PubMed]
- Heelan RT, Rusch VW, Begg CB, et al. Staging of malignant pleural mesothelioma: comparison of CT and MR imaging. AJR Am J Roentgenol 1999;172:1039-47. [Crossref] [PubMed]
- Stewart DJ, Martin-Ucar A, Pilling JE, et al. The effect of extent of local resection on patterns of disease progression in malignant pleural mesothelioma. Ann Thorac Surg 2004;78:245-52. [Crossref] [PubMed]
- Erasmus JJ, Truong MT, Smythe WR, et al. Integrated computed tomography-positron emission tomography in patients with potentially resectable malignant pleural mesothelioma: Staging implications. J Thorac Cardiovasc Surg 2005;129:1364-70. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Armato SG, Oxnard GR, MacMahon H, et al. Measurement of mesothelioma on thoracic CT scans: a comparison of manual and computer-assisted techniques. Med Phys 2004;31:1105-15. [Crossref] [PubMed]
- Armato SG, Oxnard GR, Kocherginsky M, et al. Evaluation of semiautomated measurements of mesothelioma tumor thickness on CT scans. Acad Radiol 2005;12:1301-9. [Crossref] [PubMed]
- Armato SG, Nowak AK, Francis RJ, et al. Observer variability in mesothelioma tumor thickness measurements: defining minimally measurable lesions. J Thorac Oncol 2014;9:1187-94. [Crossref] [PubMed]
- Wang ZJ, Reddy GP, Gotway MB, et al. Malignant pleural mesothelioma: evaluation with CT, MR imaging, and PET. Radiographics 2004;24:105-19. [Crossref] [PubMed]
- Eibel R, Tuengerthal S, Schoenberg SO. The role of new imaging techniques in diagnosis and staging of malignant pleural mesothelioma. Curr Opin Oncol 2003;15:131-8. [Crossref] [PubMed]
- Knuuttila A, Kivisaari L, Kivisaari A, et al. Evaluation of pleural disease using MR and CT. With special reference to malignant pleural mesothelioma. Acta Radiol 2001;42:502-7. [PubMed]
- Pass HI, Temeck BK, Kranda K, et al. Preoperative tumor volume is associated with outcome in malignant pleural mesothelioma. Journal of Thoracic and Cardiovascular Surgery 1998;115:310-7. [Crossref] [PubMed]
- Gill RR, Richards WG, Yeap BY, et al. Epithelial Malignant Pleural Mesothelioma After Extrapleural Pneumonectomy: Stratification of Survival With CT-Derived Tumor Volume. AJR Am J Roentgenol 2012;198:359-63. [Crossref] [PubMed]
- Kircheva DY, Husain AN, Watson S, et al. Specimen weight and volume: important predictors of survival in malignant pleural mesothelioma. Eur J Cardiothorac Surg 2016;49:1642-7. [Crossref] [PubMed]
- Plathow C, Klopp M, Thieke C, et al. Therapy response in malignant pleural mesothelioma-role of MRI using RECIST, modified RECIST and volumetric approaches in comparison with CT. Eur Radiol 2008;18:1635-43. [Crossref] [PubMed]
- Rusch VW, Gill R, Mitchell A, et al. A Multicenter Study of Volumetric Computed Tomography for Staging Malignant Pleural Mesothelioma. Ann Thorac Surg 2016;102:1059-66. [Crossref] [PubMed]
- Gill RR, Naidich DP, Mitchell A, et al. North American Multicenter Volumetric CT Study for Clinical Staging of Malignant Pleural Mesothelioma: Feasibility and Logistics of Setting Up a Quantitative Imaging Study. J Thorac Oncol 2016;11:1335-44. [Crossref] [PubMed]
- Chen M, Helm E, Joshi N, et al. Computer-aided volumetric assessment of malignant pleural mesothelioma on CT using a random walk-based method. Int J Comput Assist Radiol Surg 2017;12:529-38. [Crossref] [PubMed]
- Weber MA, Bock M, Plathow C, et al. Asbestos-related pleural disease: value of dedicated magnetic resonance imaging techniques. Invest Radiol 2004;39:554-64. [Crossref] [PubMed]
- Nowak AK, Francis RJ, Phillips MJ, et al. A novel prognostic model for malignant mesothelioma incorporating quantitative FDG-PET imaging with clinical parameters. Clin Cancer Res 2010;16:2409-17. [Crossref] [PubMed]
- Butchart EG, Ashcroft T, Barnsley WC, et al. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976;31:15-24. [Crossref] [PubMed]
- Bilgi Z, Colson YL. Lymphatic drainage of the pleura and its effect on tumor metastasis and spread. Türk Toraks Derneği Plevra Bülteni, 2009.
- Okiemy G, Ele N, Odzebe AS, et al. Lymphatic drainage of intertracheobronchial lymph nodes. An anatomical study by injecting diaphragmatic pleura of foetuses and adult cadavers. Le Mali Medical 2008;23:34-7. [PubMed]
- Flores RM, Akhurst T, Gonen M, et al. Positron emission tomography defines metastatic disease but not locoregional disease in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2003;126:11-6. [Crossref] [PubMed]
- Plathow C, Staab A, Schmaehl A, et al. Computed tomography, positron emission tomography, positron emission tomography/computed tomography, and magnetic resonance imaging for staging of limited pleural mesothelioma: initial results. Invest Radiol 2008;43:737-44. [Crossref] [PubMed]
- Sørensen JB, Ravn J, Loft A, et al. Preoperative staging of mesothelioma by 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography fused imaging and mediastinoscopy compared to pathological findings after extrapleural pneumonectomy. Eur J Cardiothorac Surg 2008;34:1090-6. [Crossref] [PubMed]
- Nakas A, Waller D, Lau K, et al. The new case for cervical mediastinoscopy in selection for radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2012;42:72-6; discussion 76. [Crossref] [PubMed]
- Pilling JE, Stewart DJ, Martin-Ucar AE, et al. The case for routine cervical mediastinoscopy prior to radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2004;25:497-501. [Crossref] [PubMed]
- Waller DA. The staging of malignant pleural mesothelioma: are we any nearer to squaring the circle? Eur J Cardiothorac Surg 2016;49:1648-9. [Crossref] [PubMed]
- Finn RS, Brims FJH, Gandhi A, et al. Postmortem findings of malignant pleural mesothelioma: a two-center study of 318 patients. Chest 2012;142:1267-73. [Crossref] [PubMed]
- Moser S, Beer M, Damerau G, et al. A case report of metastasis of malignant mesothelioma to the oral gingiva. Head Neck Oncol 2011;3:21. [Crossref] [PubMed]
- Sibio S, Sammartino P, Accarpio F, et al. Metastasis of pleural mesothelioma presenting as bleeding colonic polyp. Ann Thorac Surg 2011;92:1898-901. [Crossref] [PubMed]
- Tertemiz KC, Ozgen Alpaydin A, et al. Multiple distant metastases in a case of malignant pleural mesothelioma. Respir Med Case Rep 2014;13:16-8. [Crossref] [PubMed]
- Rathinam S, Waller DA. Pleurectomy decortication in the treatment of the “trapped lung” in benign and malignant pleural effusions. Thorac Surg Clin 2013;23:51-61. vi. [Crossref] [PubMed]
- Rintoul RC, Ritchie AJ, Edwards JG, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet 2014;384:1118-27. [Crossref] [PubMed]
- Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii32-40. [Crossref] [PubMed]
- Qureshi RA, Collinson SL, Powell RJ, et al. Management of malignant pleural effusion associated with trapped lung syndrome. Asian Cardiovasc Thorac Ann 2008;16:120-3. [Crossref] [PubMed]
- Hallifax RJ, Corcoran JP, Psallidas I, et al. Medical thoracoscopy: Survey of current practice-How successful are medical thoracoscopists at predicting malignancy? Respirology 2016;21:958-60. [Crossref] [PubMed]
Cite this article as: Blyth KG, Cowell GW, Bilancia R. Advances in mesothelioma imaging and implications for surgical management. Shanghai Chest 2018;2:58.


