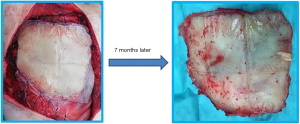
Rigid prosthesis removal following chest wall resection and reconstruction for cancer
Introduction
The most common oncologic indications for chest wall resection include primary or secondary chest wall tumours and contiguous involvement from breast or lung cancer (1). The basic principles for effective management of complex post-resectional chest wall defects are rib cage reconstruction and adequate soft tissue coverage.
Small defects, or those located posteriorly under the scapula above the fourth rib can be closed with soft tissue alone, whereas larger anterior or lateral defects need prosthetic reconstruction. A wide spectrum of synthetic mesh can be used to attain skeletal stability, composite methyl methacrylate sandwich being one of the most commonly used rigid prostheses (2).
According to le Roux and Shama, the ideal prosthetic material should offer rigidity, inertness, malleability and radiolucency (3). Although no material has been found to fulfil all the criteria, synthetic materials like Marlex mesh or Prolene are satisfactory for the reconstruction of medium-sized defects (1). For larger defects, where structural integrity is essential to avoid chest wall collapse, methyl methacrylate sandwich, silicone, Teflon, or acrylic materials have been used with good results (4). Although a plethora of synthetic materials can be utilized to stabilize the resected chest wall, there is no consensus on the most physiologic or effective substance. Wound complications such as infection, dehiscence, flap loss, and hematoma are reported to occur in up to 20% of cases (5). Nonetheless, prosthesis explants due to infected mesh are seldom reported and few data are available on the indications, technique, timing and outcome of rigid prosthesis removal (Figure 1).
This paper aimed to assess the indications for chest wall prosthesis explants, as well as surgical technique and clinical outcomes in the case of mesh infections or colonization by local recurrence.
Methods
This is an observational retrospective study conducted in accordance with the Declaration of Helsinki (6). Written informed consent was obtained from all subjects before any procedure was done. The investigators explained all the planned procedures verbally to all subjects who received and signed an information sheet to acquaint themselves with details of the planned therapeutic schedule. All patients authorized the investigators to use their data anonymously only for scientific purposes according to Italian legislation (law no. 675/1996). Data were collected prospectively and entered into our institutional general thoracic database at the point of care and reviewed and double-checked retrospectively.
A total of 166 consecutive patients underwent chest wall resection and reconstruction by rigid prosthesis (polypropylene double mesh + methyl methacrylate) over an 18-year period. Among them 10 patients (6.0%) required prosthesis removal, representing the present study population.
Demographic, clinical, pathologic and operative data and perioperative outcomes were collected: age, sex, type and extent of chest wall resection received, complications, histology of chest wall infiltrating lesion, adjuvant treatments, interval time between first procedure and prosthesis removal, cause of prosthesis removal and—in case of infection—microbiological findings, chest wall reconstruction technique following rigid prosthesis removal, procedure duration, hospital stay and overall postoperative mortality and morbidity were recorded.
Results
Five patients in the study population were male (50%); mean age was 59 years (range, 27–73 years); 3 patients (30%) received a two-rib resection; 4 patients (40%) received a three-rib resection; 3 patients (30%) received a four-rib resection; 5 cases (50%) had an associated partial sternal resection. Neoplastic disease of the chest wall was: sarcoma (4 pts: 40%); breast cancer (2 pts: 20%); other (4 pts: 40%, mesothelioma, lung cancer, thymic carcinoma, single site colonic metastasis). All 10 patients (100%) received methyl methacrylate sandwich; prosthesis coverage was obtained by muscular rotational flap in 6 patients (60%) and by direct soft tissue coverage in 4 (40%).
Three patients (30%) received postoperative radiotherapy; 1 patient (10%) received postoperative chemotherapy; 1 patient (10%) received postoperative combined radio-chemotherapy; 1 patient received preoperative combined radio-chemotherapy (10%); 4 patients (40%) did not receive any adjuvant or neoadjuvant treatments.
Mean interval time between the first procedure and prosthesis removal was 15.4 months (range, 1–161 months). Indications for prosthesis removal were infection (8 pts: 80%) or neoplastic recurrence (2 pts: 20%). In the group of infected prostheses, Staphylococcus aureus was isolated in three cases; Staphylococcus aureus and Streptococcus agalactiae in one case; Staphylococcus epidermidis in one case; Streptococcus mitis in one case; Staphylococcus not otherwise specified in one case; no isolation in one case. Reconstruction following prosthesis removal was: absorbable mesh in 3 cases (30%), no prosthesis in 4 patients (40%), other in 2 patients (20%) (1 rigid prosthesis, 1 Gore-tex® prosthesis). Mean procedure duration was 149.5 minutes (range, 39–446 minutes). The postoperative course was uneventful in 9 cases whereas 1 patient (10%) had flap dehiscence requiring reoperation. Mean total length of hospital stay was 7 days (range, 1–17 days) (Table 1).
Table 1
| Patient | Age | Sex | Treatments after 1st implant | Indication for removal | Interval time (months) | Reconstruction | Length of hospital stay (days) |
|---|---|---|---|---|---|---|---|
| 1 | 35 | Male | Radio | Recurrence | 37.1 | Gore-tex® | 6 |
| 2 | 27 | Male | Radio | Infection | 21.2 | None | 7 |
| 3 | 42 | Female | Chemoradiation | Infection | 9.1 | Vicryl | 6 |
| 4 | 65 | Male | None | Infection | 1.0 | Vicryl | 17 |
| 5 | 64 | Female | None | Infection | 9.5 | None | 1 |
| 6 | 62 | Female | None | Infection | 7.4 | None | 7 |
| 7 | 43 | Female | Radio | Recurrence | 161.3 | Rigid | 8 |
| 8 | 71 | Male | Chemoradiation | Infection | 32.9 | None | 6 |
| 9 | 73 | Male | Chemo | Infection | 80.0 | Vicryl | 9 |
| 10 | 57 | Female | None | Infection | 7.5 | None | 7 |
Discussion
Although there is some controversy as to which chest wall resections should be reconstructed, posterior defects close to the tip of the scapula, demolitions larger than 5 cm in size in any location, and most anterior defects usually require reconstruction (7). Methyl methacrylate—usually sandwiched between two layers of mesh—is one of the most widely used prostheses to provide rigidity after chest wall resection for cancer, thus avoiding flail segments which move in a paradoxical pattern with respiration, causing inefficient ventilation (8).
Although rigid prostheses provide excellent chest wall stability and a low risk of pulmonary complications, they have been associated with a greater number of wound complications. However, it is not clear whether these were related to the prosthesis itself or to the size of the chest wall defects or other factors such as the length of the operation or form of soft tissue reconstruction (5). Only 8 patients (4.8%) in our series of 166 consecutive patients treated by rigid prosthesis presented mesh infections requiring explants. In fact, chest wall infections arising in the presence of synthetic materials can be managed with appropriate incision and drainage and intravenous antibiotic therapy (9). Negative-pressure wound therapy—also called vacuum-assisted closure—may serve as a temporary measure in several cases if definitive closure or flap coverage needs to be delayed or to manage external wounds when rib cage stability has been obtained (10,11). However, vacuum-assisted closure is contraindicated over exposed blood vessels or organs and some concerns exist about its use in wounds containing malignant tumours, untreated osteomyelitis, necrotic tissue, or non-enteric and unexplored fistulae (8). The indications for prosthesis explant in our series included infected mesh exposure not amenable to sterilization, and adequate new soft tissue coverage. In any case, vacuum-assisted closure was attempted in selected cases to minimize the microbacterial load before surgical explant. Only in two cases was mesh removal due to oncologic disease recurrence incorporating the edge of the prosthesis: in one case it was a thymic carcinoma recurring 37.1 months after resection and postoperative radiotherapy; the second case was a chondrosarcoma recurring 161.3 months after resection and postoperative radiotherapy.
If the foreign material is removed at least six to eight weeks after first implant, a thick strong fibrous layer will be formed that is rigid enough to furnish chest wall stability (8,12-14): only in one of our patients did we use a new rigid prosthesis, due to an extended re-do chest wall resection for local recurrence whereas soft mesh or no mesh were used in all other cases.
In conclusion, composite rigid mesh removal is indicated in case of prosthetic infection, or rarely in case of chest wall recurrence at the edges of the prosthesis. Reconstruction after prosthesis removal may require soft mesh but very often no new prosthesis is needed due to fibrosis stabilizing the chest wall. This is a safe and effective procedure, generally with an uneventful postoperative course.
Acknowledgments
The Authors thank Anne Prudence Collins for editing the English text.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.08.02). LS serves as an unpaid editorial board member of Shanghai Chest from Jul 2017 to Jun 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained; being a retrospective study on patients already allowing the use of their data, no formal ethical approval was needed but formal authorization was obtained by each patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mansour KA, Thourani VH, Losken A, et al. Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg 2002;73:1720-5; discussion 1725-6.
- McCormack PM. Use of prosthetic materials in chest-wall reconstruction. Assets and liabilities. Surg Clin North Am 1989;69:965-76. [Crossref] [PubMed]
- le Roux BT, Shama DM. Resection of tumors of the chest wall. Curr Probl Surg 1983;20:345-86. [Crossref] [PubMed]
- Graeber GM, Langenfeld J. Chest wall resection and reconstruction. In: Franco KL, Putman JR. editors. Advanced therapy in thoracic surgery. London: BC Decker, 1998:175-85.
- Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 2006;81:279-85. [Crossref] [PubMed]
- World Medical Association Declaration of Helsinki Ethical principles for medical research involving human subjects Helsinki 1964, amended in Tokyo 1975, Venice 1983, Hong Kong 1989, South Africa 1996, Edinburgh 2000, Washington 2002, Tokyo 2004, Seoul 2008, Fortaleza 2013.
- Deschamps C, Tirnaksiz BM, Darbandi R, et al. Early and long-term results of prosthetic chest wall reconstruction. J Thorac Cardiovasc Surg 1999;117:588-91; discussion 591-2. [Crossref] [PubMed]
- Skoracki RJ, Chang DW. Reconstruction of the chestwall and thorax. J Surg Oncol 2006;94:455-65. [Crossref] [PubMed]
- Kroll SS, Walsh G, Ryan B, et al. Risks and benefits of using Marlex mesh in chest wall reconstruction. Ann Plast Surg 1993;31:303-6. [Crossref] [PubMed]
- Obdeijn MC, de Lange MY, Lichtendahl DH, et al. Vacuum-assisted closure in the treatment of poststernotomy mediastinitis. Ann Thorac Surg 1999;68:2358-60. [Crossref] [PubMed]
- Fleck TM, Fleck M, Moidl R, et al. The vacuum-assisted closure system for the treatment of deep sternal wound infections after cardiac surgery. Ann Thorac Surg 2002;74:1596-600; discussion 1600. [Crossref] [PubMed]
- Petrella F, Radice D, Borri A, et al. Chest wall resection and reconstruction for locally recurrent breast cancer: From technical aspects to biological assessment. Surgeon 2016;14:26-32. [Crossref] [PubMed]
- Spaggiari L, Galetta D, Veronesi G, et al. Superior vena cava replacement for lung cancer using a heterologous (bovine) prosthesis: preliminary results. J Thorac Cardiovasc Surg 2006;131:490-1. [Crossref] [PubMed]
- Borri A, Leo F, Veronesi G, et al. Extended pneumonectomy for non-small cell lung cancer: morbidity, mortality, and long-term results. J Thorac Cardiovasc Surg 2007;134:1266-72. [Crossref] [PubMed]
Cite this article as: Petrella F, Casiraghi M, Mariolo AV, Diotti C, Spaggiari L. Rigid prosthesis removal following chest wall resection and reconstruction for cancer. Shanghai Chest 2018;2:64.