Induction radiotherapy and mesothelioma surgery
Introduction
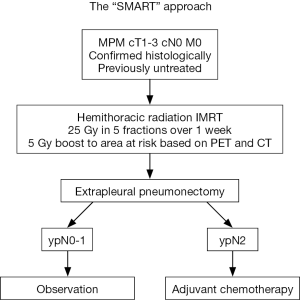
Many previous attempts at providing aggressive treatment for mesothelioma have failed due to the inability of patients to successfully complete the intended protocol with three therapeutic modalities. Upon reviewing our experience with induction chemotherapy followed by surgery and adjuvant hemithoracic radiation, we observed that radiation correlated with the best response due to the excellent local control. In order to enhance the impact of radiation on disease control, reduce risk of tumour spillage, and potentially improve survival while ensuring that all patients complete the hemithoracic radiation component, the Surgery for Mesothelioma After Radiation Therapy (SMART) protocol was developed. This protocol is the first to include ipsilateral hemithoracic intensity-modulated radiation therapy (IMRT) prior to surgery. The SMART protocol entails irradiation to the entire ipsilateral hemithorax with a concomitant boost to volumes at high risk based on computed tomography (CT) and positron emission tomography (PET) scan findings in 5 daily fractions over 1 week. In order to prevent toxic radiation pneumonitis from developing in the ipsilateral lung, an extra-pleural pneumonectomy (EPP) is performed within two weeks of completion of the radiation therapy. For patients who are found to have ypN2 disease on final pathology, adjuvant chemotherapy consisting of cisplatin and an antifolate agent (pemetrexed or raltitrexed) is offered (Figure 1). The administration of a third therapy in patients with ypN2 disease has been difficult. Therefore, currently patients generally do not receive adjuvant chemotherapy regardless of the nodal status on final pathology. Patients are carefully monitored after surgery and started on chemotherapy with cisplatin—pemetrexed only when evidence of recurrence is diagnosed on CT scan. Chemotherapy is thus easier to administer and the CT scan provides a target to evaluate response. The new grouping of N2 into N1 disease in the 8th edition of the TNM system did not impact our practice as patients with clinical N1 and N2 disease were excluded from SMART and patients are being observed after surgery despite the presence of ypN1 or ypN2 disease on final pathology.
Induction radiotherapy
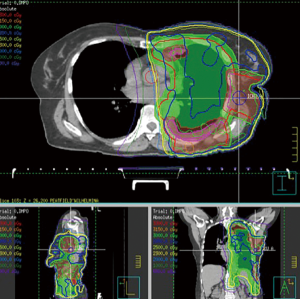
From a radiation perspective, the clinical target volume (CTV) is defined as the ipsilateral hemithorax, from the thoracic inlet down to the diaphragmatic insertion, including biopsy and drainage tract sites. The gross tumour volume (GTV) is defined as any tumor seen on CT and FDG-PET. The intended dose to the CTV is 25 Gy in 5 daily fractions over 1 week with a concomitant boost of 5 Gy to the GTV and tract sites. A multi-beam intensity modulated radiation therapy (IMRT) technique is used (Figure 2). As the diagnosis of mesothelioma can be quite difficult, all patients have a histological diagnosis of mesothelioma made by pleuroscopy or core biopsy prior to coming to starting the SMART protocol. Due to high rates of tumour ingrowth into the port sites, the number of port sites should be limited to one for diagnostic purpose and the chest wall, with a diameter of 6 cm, surrounding each port site is boosted with radiation. Early on in our experience, we observed two patients with biphasic disease who developed recurrence in the retroperitoneal lymph nodes and, therefore, since 2012, the radiation field has been refined to also cover the lymphatic drainage from the diaphragm to the upper retroperitoneum.
Patient selection
Patients eligible for this protocol are those with histologically proven epithelioid mesothelioma, clinical stage T1-3N0M0, based on CT chest and abdomen, PET-CT scan, and brain magnetic resonance imaging or CT. Endobronchial ultrasound (EBUS) and transbronchial needle aspiration biopsy (TBNA) of mediastinal and hilar lymph nodes have been included as part of the staging procedure more recently. Patients must have a forced expiratory volume in one second greater than 40% predicted and a diffusing capacity for carbon monoxide >45% predicted, with an Eastern Cooperative Oncology Group performance status of 0–2. From a clinical perspective, much consideration is given to patients who present with chest pain unrelated to previous thoracoscopy incisions because, although not always obvious on imaging, they often have chest wall invasion and are not ideal candidates due to the difficulty in performing an R0-1 resection. Also, patients must be committed to completing the treatment as the surgery must be performed once the radiation commences due to the risk of fatal pneumonitis if the lung is not resected. Adequate social support is also important since the protocol has a lengthy recovery time. From an imaging perspective, patients with chest wall, peritoneal, or mediastinal extension are not offered surgery. Also, upon reviewing our results, tumour thickness was found to correlate with gross tumor volume and has a significant impact on overall survival (HR 1.12, P=0.02) and disease-free survival (HR 1.13, P=0.01) as well as epithelioid histologic subtype (HR 0.25, P<0.0001) and pN2 status (HR 2.15, P=0.02) (1). This parameter is increasingly used as a selection criterion, particularly if the bulk of disease is located on the diaphragm.
EPP
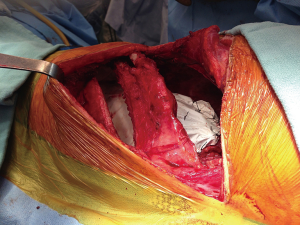
As mentioned above, within two weeks of completing radiation therapy, EPP is performed. The average time between the end of radiation and surgery has been 5 days, ranging between 2 and 12 days. As per the standard protocol for this surgery, en-bloc resection of the lung, parietal pleura, ipsilateral diaphragm and ipsilateral pericardium is completed. The resection begins with a large posterolateral incision and resection of the fifth or sixth rib to access the extra-pleural plane. Resection of the fifth rib provides better access to the apex of the pleural cavity and is selected when we anticipate difficult dissection around the thoracic inlet, otherwise the sixth rib is removed. Using a combination of blunt and sharp extra-pleural dissection, the specimen is slowly mobilized. Once the access from the current thoracotomy becomes limited, particularly in the posterior costodiaphragmatic recess, a second thoracotomy is performed in the eighth intercostal space (Figure 3). This second thoracotomy performed through the same skin incision allows increased visualization of the hemidiaphragm, especially posteriorly along the chest wall and in the area of the ipsilateral crus. Also, on the right side, the lower thoracotomy allows improved visualization for sharp dissection of the diaphragm along the inferior vena cava. The phrenic veins can easily be localized underneath the diaphragm and ligated through this second incision. Once the specimen is removed, both the diaphragm and pericardium are reconstructed using Gore-Tex™ mesh (1 mm for diaphragm and 0.1 mm for pericardium). Two Gore-Tex™ meshes are stapled together to have adequate length (Figure 4). The diaphragmatic mesh is attached with interrupted stitches along the inferior part of the pericardium and the 9th intercostal space. Posteriorly, the mesh is also fixed by stitch on the spine at the level of T8 or T9. The pericardial mesh is attached by interrupted stitches to the anterior, inferior and posterior part of the pericardium (Figure 5). In some patients with early disease the pericardium is not invaded and the pleura can be stripped without resection, but all patients have resection and reconstruction of the diaphragm. Care is taken to avoid any impingement on the inferior vena cava on the right side or the esophagus and aorta on the left side. The pericardial mesh is kept loose to avoid any cardiac constriction postoperatively. If a large part of the pericardium is removed on the left side, the pericardium is often not reconstructed.
Coverage of the bronchial stump is performed using posterior pericardium, as previously described on the right and the left side (2). The posterior pericardium is easily mobilized to cover the bronchial stump on the left. On the right side, the posterior pericardium is opened behind the right pulmonary veins and dissected off the wall of the left atrium along the oblique sinus. The pericardium is then freed from the pulmonary artery stump and the superior vena cava along the transverse sinus, allowing the posterior pericardium to be fully mobilized and attached to the structures behind the bronchial stump, such as the vagus nerve, the edge of the esophageal muscle and the azygos vein. This technique allows the bronchial stump to be “mediastinalized” and well protected. In our experience, the posterior pericardium could be used to cover the bronchial stump in over 90% of the time on the right and the left side. Should this technique not be feasible, omentum or thymus gland is used.
Post-operative management
Post-operatively, patients are monitored in a step-down unit for at least the first 48 hours after surgery. Some degree of hypotension is common, and usually managed with fluid replacement. Unlike the traditional dogma of fluid-restricting patients who have undergone pneumonectomy, in the early post-operative period after EPP judicious use of intravenous fluids is prescribed due to the large fluid shifts associated with the extensive dissection of this procedure. Occasionally, patients have to remain on low doses of noradrenaline for a few hours after the end of the surgery until the optimal fluid balance is achieved. The chest tube remains in place for two or three days (occasionally longer), predominantly to observe the character of output and monitor for bleeding. Prophylactic antibiotics are continued until the chest tube is removed, in an attempt to prevent the devastating complication of infection associated with a chest tube in proximity to mesh prostheses. Although nasogastric tubes are frequently used intraoperatively to aid in identification of the esophagus, they are removed prior to the completion of the procedure. Patients remain nil per os for the first 24 hours, then the diet is slowly advanced as bowel function returns. Due to the diaphragmatic reconstruction and often intraperitoneal exposure, patients are prone to ileus and all attempts should be made to prevent dilatation of the stomach and vomiting in the early post-operative period, in order to prevent undue strain on the diaphragmatic repair, decrease pain and attempt to prevent aspiration. Bloodwork is tested twice daily for the first few days, including complete blood count, electrolytes and extended electrolytes, liver enzymes and creatinine kinase. Creatine kinase is checked daily as there is frequently a component of rhabdomyolysis in these patients due to the length of time spent in the lateral decubitus position intra-operatively. Daily chest X-rays are performed.
Results
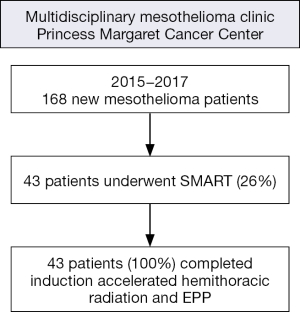
At our institution, all patients referred with a diagnosis of mesothelioma are considered for the SMART protocol. Reasons for exclusion include predominantly advanced stage mesothelioma (54%), poor performance status with comorbidities (35%), and sarcomatoid histological subtype (10%). Over the past 3 years between 2015 and 2017, 168 new patients with malignant pleural mesothelioma (MPM) were seen in our multidisciplinary clinic at Princess Margaret Cancer Centre and 43 of them (26%) underwent SMART (Figure 6). Induction accelerated hemithoracic radiation and EPP were completed as planned in all patients. Patients with biphasic disease are generally excluded from this protocol. However, about 20% of the patients have a postoperative diagnosis of biphasic disease that was not detected preoperatively on the biopsy.
Overall, the median survival as an intention-to-treat analysis is 28.3 months (3). This is a dramatic improvement from our previous experience with induction chemotherapy followed by EPP and adjuvant hemithoracic radiation as the intention-to-treat analysis in this cohort of patients was 14 months (4). Other prospective multimodality therapy trials have reported an intention-to-treat survival ranging between 16.8 and 25.5 months (5). The survival in multimodality trial reached 23.9 to 39.4 months in patients who completed the hemithoracic radiation after EPP (5). Hence, the outcome of 28.3 months falls within the range of survival for patients who completed the adjuvant radiation as part of the trimodality approach in previous trials.
Our results have been particularly encouraging in patients with epithelioid mesothelioma. The overall median survival for this group of patients is 36 months with a disease-free survival of 30 months. However, the median survival ranges from 18 months when tumor volume was greater than 500 cm3 to 51 months when tumor volume was less than 500 cm3 (3). These results compare favorably with other reports in the literature. The median survival for epithelial mesothelioma and tumor volume of less than 500 cm3 ranges between 24 and 34 months, while the median survival ranges between 8 and 17 months when the tumor volume of epithelial tumor is greater than 500 cm3 in the literature (5-7).
Local control with induction accelerated radiation was very good and provided similar results to adjuvant high dose normofractionated hemithoracic radiation. Locoregional recurrence defined by recurrence along the chest wall or mediastinum was observed in 22% of the patients. Biphasic tumor and bulky epithelial mesothelioma ypT4N2 were risk factors for local relapse after induction hypofractionated radiation and EPP (8).
Surgical complications
In the pilot study of 25 patients, 52% of patients developed grade 3 (G3) complications or higher. The most common complication was atrial fibrillation, at a rate of 20%. Over time, though, the rate of G3+ complications have decreased as experience builds, and currently less than 20% of patients with epithelial mesothelioma develop a major complication with the exception of atrial fibrillation. Atrial fibrillation is the only complication that has remained frequent at about 20% and has not changed over time. Other complications included thromboembolic events, wound infection, chylothorax, hemothorax, wound dehiscence, renal dysfunction, pneumonia and empyema (Table 1). There have been no patients who developed a broncho-pleural fistula. The rate of surgical complications after induction hemithoracic radiation was similar to those after induction chemotherapy (9). The 90-day mortality rate was 3.8% after induction hemithoracic radiation compared to 6.3% after induction chemotherapy.
Table 1
| Complications | Total number of patients (N=106) |
|---|---|
| Grade 3+ complications | 40 (38%) |
| Atrial fibrillation | 23 |
| Wound dehiscence/infection | 6 |
| Empyema | 6 |
| Venous thromboembolism | 5 |
| Pneumonia | 5 |
| Hemothorax | 3 |
| Chylothorax | 2 |
| Diaphragmatic dehiscence | 2 |
| Clostridium difficile colitis | 2 |
| Platypnea-orthodeoxia syndrome | 1 |
| Renal dysfunction | 1 |
EPP, extra-pleural pneumonectomy.
Early on in our experience, we found that 16% of the patients experienced partial wound dehiscence at about a month after surgery. We therefore modified our closure technique and replaced vicryl with Ticron™ (Covidien, Ontario, Canada) sutures for muscular and subcutaneous layers, and resection of port sites is performed only where gross disease is present. In an attempt to seal the pleural space and prevent seroma formation, the intercostal space at the level of the resected port sites are also closed with a small patch of 1 mm Gore-Tex™ mesh (W.L. Gore and associates, Flagstaff, AZ, USA).
One patient developed pulmonary emboli while in hospital postoperatively, while 3 developed venous thromboembolic events after discharged from hospital. We therefore have modified our algorithm and currently discharge all of our patients on prophylactic low molecular weight heparin for 4–6 weeks after surgery. Although the number is small, this changed seems to have reduced the risk of venous thromboembolic problem after discharge from hospital in our more recent experience.
Six out of 106 patients undergoing SMART (5.7%) have developed empyemas requiring open thoracostomies, none of whom had a broncho-pleural fistula. Two of the six patients received multiple cycles of chemotherapy before being referred to us and proceeded to induction hemithoracic radiation as part of the SMART protocol. The rate of empyema was therefore significantly higher in patients who had chemotherapy before SMART (2 out of 5 patients, 40%) compared to those who had SMART as their initial treatment (4 out of 101 patients, 4%) (P=0.02).
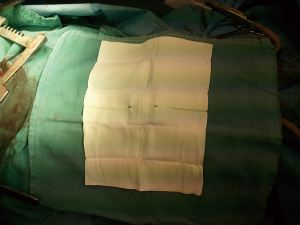
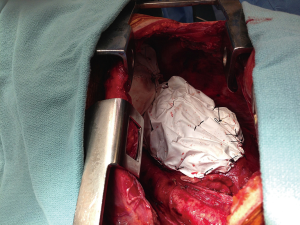
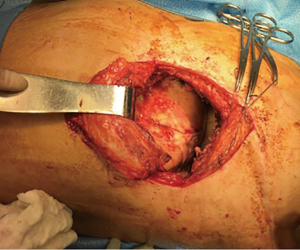
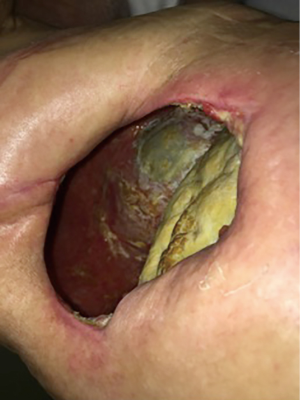
Four patients with empyema developed a wound infection during their postoperative recovery that was treated with antibiotics and local therapy such as vacuum dressing, but eventually progressed to infect the pleural space and were taken to the operating room for creation of an open window thoracostomy 2 to 12 months after their EPP. Two of these patients developed major recurrent bleeding from the pulmonary artery on the left side or the right atrium on the right side despite the open window. The second patient was treated with muscle and omental flap mobilization to cover the area of recurrent bleeding from the right atrium, allowing healing and eventually closure of the open window thoracostomy. The remaining two patients had an uncomplicated initial admission but presented five weeks post-operatively with fever, shortness of breath and increased fluid in the pneumonectomy space (Figure 7). The fluid was sampled and grew bacteria, therefore they were taken to the operating room for creation of a window (Figure 8). Both were discharged home with packing (Figure 9) and plan to close the window. The diaphragmatic and pericardial meshes were generally removed at the time of the open window surgery, including one patient within 6 weeks of the EPP and no dehiscence of abdominal content or cardiac rotation was observed.
Conclusions
Induction hemithoracic IMRT followed by EPP has shown promising results in selected patients with mesothelioma. This protocol should be considered for patients with epithelioid mesothelioma and good performance status, and especially for those with low-volume of disease, as significantly improved long-term survival is possible in these groups of patients. Refinements in radiation technique may allow preservation of the underlying lung and combination of the SMART approach with extended pleurectomy-decortication in the future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (David Waller and Annabel Sharkey) for the series “Mesothelioma Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.07.03). The series “Mesothelioma Surgery” was commissioned by the editorial office without any funding or sponsorship. MDP reports personal fees from AstraZeneca, personal fees from Bayer, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- de Perrot M, Dong Z, Bradbury P, et al. Impact of tumour thickness on survival after radical radiation and surgery in malignant pleural mesothelioma. Eur Respir J 2017;49. [PubMed]
- de Perrot M. Use of the posterior pericardium to cover the bronchial stump after right extrapleural pneumonectomy. Ann Thorac Surg 2013;96:706-8. [Crossref] [PubMed]
- Perrot M, Wu L, Wu M, et al. Radiotherapy for the treatment of malignant pleural mesothelioma. Lancet Oncol 2017;18:e532-42. [Crossref] [PubMed]
- de Perrot M, Feld R, Cho BC, et al. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:1413-8. [Crossref] [PubMed]
- Gill RR, Richards WG, Yeap BY, et al. Epithelial malignant pleural mesothelioma after extrapleural pneumonectomy: stratification of survival with CT-derived tumor volume. AJR Am J Roentgenol 2012;198:359-63. [Crossref] [PubMed]
- Rusch VW, Gill R, Mitchell A, et al. A Multicenter Study of Volumetric Computed Tomography for Staging Malignant Pleural Mesothelioma. Ann Thorac Surg 2016;102:1059-66. [Crossref] [PubMed]
- Pass HI, Temeck BK, Kranda K, et al. Preoperative tumor volume is associated with outcome in malignant pleural mesothelioma. J Thorac Cardiovasc Surg 1998;115:310-7. [Crossref] [PubMed]
- de Perrot M, Feld R, Leighl NB, et al. Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2016;151:468-73. [Crossref] [PubMed]
- Mordant P, McRae K, Cho J, et al. Impact of induction therapy on postoperative outcome after extrapleural pneumonectomy for malignant pleural mesothelioma: does induction-accelerated hemithoracic radiation increase the surgical risk? Eur J Cardiothorac Surg 2016;50:433-8. [Crossref] [PubMed]
Cite this article as: Donahoe LL, Cho BJ, de Perrot M. Induction radiotherapy and mesothelioma surgery. Shanghai Chest 2018;2:66.