
Pros-cons debate about the role and evolution of biportal video-assisted thoracoscopic surgery
Historical perspectives
Since the first video-assisted thoracoscopic surgery (VATS) lobectomy performed by Roviaro more than 20 years ago (1), this challenging surgical procedure has obtained an increasing success due to its attractiveness as a minimally invasive modality with a better outcome compared to ‘open-procedures’ in terms of fewer postoperative complications, reduced chest drainage duration and, consequently, shorter length of stay (2-6).
However, the hypothetical reduction of local recurrence of disease and the unknown effects on long term survival represent two VATS lobectomy’s dilemmas that remain unsolved until now even if a recent meta-analysis reported that VATS lobectomy is comparable with open surgery to treat patients with early-stage non-small cell lung cancer. More information regarding oncologic efficacy of this procedure needs to be accumulated in order to gain a wider acceptance of this procedure (6-11).
Despite this, Mahtabifard and McKenna affirm that VATS lobectomy, if performed by skilled surgeons, can offer the same or better complications rate as well as the same safety profile if compared to open surgery (12).
So, attempts to decrease the size of the working port, the diameter of instruments as well as the number of incisions had been made at the same rate as VATS procedures improves (13-16).
An initial report about the feasibility of biportal VATS lobectomy by Borro et al. (16) suggests us that third port is not necessary.
Current perspectives
The first definition of VATS lobectomy goes back to 2007 when a prospective multicentre study, CALGB 39802, defined a VATS lobectomy as a procedure performed with videoscopic guidance and anatomic vascular, bronchial and lymphatic dissection using three or four ports and without rib spreading (17).
Even if there is no accordance with the existence, to date, of a standard technique of VATS lobectomy, most centres use a utility incision of about 3–5 cm generally positioned anteriorly. A great number of surgeons then add two other ports (one for the optics and another at posterior level (4).
Gossot et al., in 2009, described a totally endoscopic major pulmonary procedure for early stage bronchial carcinoma using three incisions with a minithoracotomy for the extraction of the lobe (18).
The work of D’Amico and co-author is the first where it is described a large number of VATS procedures performed by a biportal approach that it is revealed as a safe and versatile procedure (19). Finally, Gonzalez reported in 2011 the first uniportal VATS lobectomy, still in its infancy yet, which becomes a milestone in the development history of VATS lobectomy (20).
Recently, we have assisted to the wide acceptance and diffusion of videothoracoscopic surgical interventions due primary to its undeniable advantages (e.g., less pain, better preserved lung function and faster discharge) (21-25).
Whereas the most common modality of minimally invasive thoracic surgery is the three-ports approach (endoscopic hole, main and second operational hole), we have to consider that the second operational hole is usually done between the posterior axillary line and the infrascapular line. That is an area very rich of vessels, then we could have a difficult haemostasis process in event of blood loss (26). Also, the second operational hole is often the cause of postoperative pain due to rotational movement of the trocar during the operation that could cause injury of the intercostal nerve (27,28). Thus, removal of this operative hole could reduce postoperative pain and difficult haemostasis.
Biportal VATS has been performed in our department since January 2014 for the treatment of early stage lung cancer.
As revealed in this review, VATS with a single utility port had different advantages such as lower length of hospitalization (P<0.05), lower duration of chest tube (P<0.05), and less pain (P<0.05) compared with three- or four-port groups. As a consequence, the higher operative skills required to perform an operation through a single hole could to increase the surgical time. Pulmonary anatomy has to be well known and the very common interferences between the instruments passed through a single operation port have to be overcome.
We suggest that the use of a rotatable table associated with a double joint thoracoscopic instrument could help to perform a surgical biportal intervention with less difficulties. In certain cases the thoracoscope could be inserted into the second hole, whereas the instrument is inserted though the camera-port.
We strongly believe that biportal VATS is a safe and feasible procedure but it is important to consider some tricks like the sharp separation by scalpel that would be recommended instead of monopolar incision. Also, phrenic and recurrent laryngeal nerves should be carefully protected when mediastinal lymph nodes are dissected (right side: stations 2, 3, 4, 7, 8, 9; left side: stations 5, 6, 7, 8, 9).
Methods
We started the program of VATS lobectomy in May 2012. Since then, VATS lobectomy has been applied in our Unit more and more widely up to the numbers of 2016, when VATS lobectomy versus open lobectomy had a ratio of 83% vs. 27%. Its development at our Department can broadly be divided into the phases: 4-, 3- and 2-port approach.
In May 2012 we performed the first VATS lobectomy using a 4-port approach; it was a right upper lobectomy and operative time was 160 minutes. From then, we performed other 42 procedures with 4-port approach.
In this process, we tried consciously to transform the typical four or three ports into two ports: in fact, since 2014 we have performed a consecutive 3-port approach in 56 patients and finally 2-port in 302.
Usually we place the camera-port in the 8th intercostal space in the posterior axillary line and the utility incision (4–5 cm) in the 4th–5th intercostals space in the anterior axillary line. We introduce through the camera-port a 12-mm trocar for the 10 mm-30° camera and we prefer to staple the pulmonary vein first and then the artery, wherever possible, and sampling of the different mediastinal lymph nodes stations, according to ESTS guidelines. During the procedure, in case of necessity, two instruments plus the thoracoscopic optic are positioned through the camera port. Usually, we introduce the stapler for suture/section of the pulmonary vessels and bronchus through the camera port in the upper lobectomy; through the utility incision in the lower lobectomy. The lobe is placed into a bag and removed through the utility incision and a single 28Ch in size tube is placed at the end of the surgery. The drainage system is taken under a standard or digital suction for the first 24 hours.
Almost all patients were subjected to preoperative pulmonary function tests, total body FDG PET/TC and fibrobronchoscopy.
In order to establish the difference of outcome and, subsequently, pro and cons of 2 portal VATS approach we have compared our experience in terms of VATS lobectomy done via 4-, 3- and 2-port approach. Thus, we have compared type of intervention, operative time, number of lymph nodes retrieved, complication and conversion rate, postoperative length of stay, chest tube duration, pStage and postoperative pain grade.
Operative technique: biportal VATS
Under general anaesthesia a double lumen tube was placed with the help of a bronchoscopic guidance. After that a single lung ventilation was performed and the patient was kept in full lateral decubitus position (clasp-knife position). Positioning the patient in this way (slight flexion of the table at the level of the mid-chest) allows splaying of the ribs to improve exposure in the absence of rib spreading. It is essential that a total lung collapse is carried out correctly.
Biportal VATS requires two incisions. The first incision, 1.5 cm sized, is placed at level of 8th–9th intercostal space along the posterior axillary line. It is called ‘camera-port’ because usually we introduce through it the thoracoscope. The second incision, also called ‘utility-incision’, is performed at 4th–5th intercostals space along the anterior axillary line. This incision, 4–5 cm sized, provides access for hilar dissection and it is placed below the breast and the pectoralis major muscle because of the greater width of the intercostals spaces in this area. An incision protector sleeve not ever is used instead of silk stitches.
Biportal VATS follows the general principles of oncologic major pulmonary resections. Thus, individual dissection of veins, bronchus and arteries associated with mediastinal lymph node dissection is performed.
Instruments should be long and curved to permit the insertion of two or three instruments simultaneously. Then, we use a combination of traditional and thoracoscopic equipment.
A 12-mm trocar for the 10 mm-30° camera is usually inserted through the lower incision and the first action is to inspect the pleural cavity in order to rule out the presence of pleural metastasis and hilar invasion. Also the presence of adhesions should be carefully checked and, eventually, detached.
Pulmonary vein was firstly isolated and ligated anterior to the hilum or the lower pulmonary ligament. Subsequently, bronchial branch and the artery were addressed using a surgical stapling instrument. Finally, the incomplete lobar fissure was stapled.
It is important to consider that to make an anatomical operation though two incisions, the camera and the instruments are exchanged from one incision to the other. For the lower and middle lobectomies it is usually unnecessary to make this change but for upper lobectomies the endostaplers should be inserted from the camera-port.
A vascular clip or Hem-o-lok® could be used in the case of small vascular rupture.
Mediastinal lymph node dissection, if initially performed, could help to dissect and identify hilar structures. In every case the extent of the dissection should be not inferior to conventional thoracotomy. So, on the right side stations 2, 3, 4, 7, 8 and 9 were dissected whereas on the left side stations 5, 6, 7, 8 and 9.
Normally, one 28Ch Intrathoracic drainage tube is inserted after the operation.
From October 2017 we have introduced a 3D thoracoscope. According to literature (29,30) we reckon that the use of 3D technology doesn’t add more advantages in terms of operative characteristics and safety profile for radical resection of NSCLC.
Statistical analysis
Pearson’s χ2 test was used to analyze the baseline characteristics and t-test was used to compare the clinical parameters between both groups. Data were expressed as the mean ± standard deviation and a P value <0.05 was considered statistically significant. To adjust for multiple testing, a Bonferroni-type procedure has been used.
Comparison of VATS effects on clinical outcome was implemented using a Propensity Score (PS) weighting approach, in order to control for potential confounders. Variable included in PS model were all the individual characteristics observed before the surgical procedure. PS values were estimated using the Non-Parametric Covariates Balancing Propensity Score (NPCBPS) technique, a non-parametric empirical likelihood based approach that estimates individual weights representing the inverse stabilizing PS and contemporarily maximizes the balance of baseline characteristics (Table 1).
Table 1
| Variables | 2-port [2014–2017] | 3-port [2013] | 4-port [2012] | P value | ||
|---|---|---|---|---|---|---|
| 4P vs. 3P | 3P vs. 2P | 4P vs. 2P | ||||
| Sex | ||||||
| M | 214 | 40 | 30 | 1 | 1 | 1 |
| F | 88 | 16 | 12 | |||
| Age (mean value ± SD) | 68.5±8.8 | 67±10 | 66.8±7.8 | 1 | 1 | 1 |
| Histology | ||||||
| Adenocarcinoma | 180 | 32 | 24 | 1 | 1 | 1 |
| Squamous cell carcinoma | 61 | 8 | 6 | 1 | 1 | 1 |
| Carcinoid | 23 | 5 | 3 | 1 | 1 | 1 |
| Metastasis | 17 | 0 | 3 | 1 | 1 | 1 |
| Other | 21 | 11 | 6 | 1 | 0.096 | 0.072 |
| Type of intervention | ||||||
| RUL | 100 | 20 | 13 | 1 | 1 | 1 |
| RML | 17 | 3 | 7 | 1 | 1 | 0.276 |
| RLL | 73 | 13 | 9 | 1 | 1 | 1 |
| LUL | 66 | 8 | 3 | 1 | 1 | 0.216 |
| LLL | 46 | 12 | 10 | 1 | 1 | 1 |
| Overall | 302 | 56 | 42 | |||
| Time (mean value ± SD) | 137±35 | 134.6±34.4 | 123±19.4 | |||
| Lymph node (mean value ± SD) | 11±4.5 | 12.24±5.9 | 8.67±3.3 | |||
| Complications | ||||||
| Number | 47 | 7 | 2 | |||
| Conversion | 26 | 11 | 9 | |||
| Chest tube duration (days, mean value ± SD) | 4.29±1.88 | 4.49±1.65 | 5.66±1.8 | |||
| Length of hospital stay (mean value ± SD) | 6±2.5 | 5.92±2 | 8±2.8 | |||
| Pain (VAS score, mean value ± SD) | 1.19±0.46 | 2.28±0.89 | 2.47±1.19 | |||
| pStage | ||||||
| IA | 177 | 37 | 30 | |||
| IB | 50 | 4 | 5 | |||
| IIA | 29 | 7 | 4 | |||
| IIB | 21 | 2 | 1 | |||
| IIIA | 5 | 6 | 2 | |||
Outcome analysis was performed with univariate weighted regression models, in order to assess how type of VATS differently impact on clinical outcomes. Generalized Linear Model (GLM) with different response distributions and different link functions were used for the analysis. In particular, gamma distribution with inverse link function for the time of surgical procedure in minutes, Poisson distribution with logarithmic link function for number of retrieved lymph nodes, days of chest tube duration and days of hospital stay, normal distribution with identity link function for pain, binomial distribution with logit link function for occurrence of complications and multinomial logistic regression for pStage. Differences were evaluated with pairwise comparison, i.e., 3P vs. 2P, 4P vs. 2P and 4P vs. 3P. Point estimates of outcome differences with relative 95% confidence intervals are also reported.
The R System and IBM SPSS 19.0 statistical softwares were used for data analysis.
Results
Demographic information is presented in Table 2. Mean age of 284 men and 116 women was 68.11±8.89 years. Pre-operative FEV1 was, on average, 87.86%±18.51% of predicted with a range of 47% to 157%.
Table 2
| Characteristic | Value |
|---|---|
| Sex | |
| M | 284 |
| F | 116 |
| Age (years, mean value ± SD) | 68.11±8.89 |
| FEV1 pre-op (%, mean value ± SD) | 87.86±18.51 |
| Histology | |
| Adenocarcinoma | 236 |
| Squamous cell carcinoma | 75 |
| Carcinoid | 31 |
| Metastasis | 20 |
| Other | 38 |
| Type of intervention | |
| RUL | 133 |
| RML | 27 |
| RLL | 95 |
| LUL | 77 |
| LLL | 68 |
| Overall | 400 |
| Time (min, mean value ± SD) | 113.08±33.7 |
| Lymph node (number, mean value ± SD) | 10.66±4.68 |
| Complications | |
| Overall | 56 |
| Conversion | 46 |
| Chest tube duration (days, mean value ± SD) | 4.47±1.9 |
| Length of hospital stay (days, mean value ± SD) | 5.96±2.66 |
| Pain (VAS score, mean value ± SD) | 1.48±0.83 |
| pStage | |
| IA | 244 |
| IB | 59 |
| IIA | 40 |
| IIB | 24 |
| IIIA | 33 |
RUL, right upper lobectomy; RML, right middle lobectomy; RLL, right lower lobectomy; LUL, left upper lobectomy; LLL, left lower lobectomy.
Thoracoscopic lobectomy was successfully performed in 354 out of 400 patients (conversion rate, 11.5%). Of the 46 conversions, 27 were for haemorrhage (controlled thoracoscopically during conversion), 15 for dense hilar adenopathy (benign) or incomplete fissures, 2 for oncologic concern and 2 for bronchial complications. All conversions were performed with haemodynamic stability and no further sequelae after conversion. For this reason we have anyway considered every converted operation as a VATS surgical procedure. Anatomic resections included all lobes; Thoracoscopic pneumonectomy was not performed. Mean operative time was 113.08±33.7 minutes.
All resections were considered R0: negative surgical margins and all pathologic lymph node resected. Mean lymph nodes dissected were 10.66±4.68. Pathologic analysis included adenocarcinoma in 236 patients (59%), squamous cell carcinoma in 75 patients (18.8%), carcinoid in 31 patients (7.8%), metastasis in 20 patients (5%) and other benign or malignant conditions in 38 patients (9.5%). For the 342 patients with NSCLC, pathologic analysis demonstrated stage IA in 244 patients (71.3%), stage IB in 59 patients (17.3%), stage IIA in 40 patients (11.7%), stage IIB in 24 patients (7%) and stage IIIA in 33 patients (9.6%). The patients with stage IV disease that had previously undergone metastasectomy for isolated cerebral metastasis were excluded from thoracoscopic approach.
The mean chest tube duration was 4.47±1.9 days and the mean length of hospitalization was 5.96±2.66 days. The operative and perioperative (30 days) mortality were 0% and 4%, respectively.
Postoperative pain was, on average, 1.48±0.83 according to VAS score. The main components of multimodal analgesia are: NSAIDs (nonsteroidal anti-inflammatory drugs), paracetamol, opioids and regional analgesia [thoracic paravertebral block (TPVB)]. The goal is to take advantage of several analgesia techniques to minimize the consumption of opioids.
Main postoperative complications (Table 3) included atrial fibrillation (25%), prolonged air leak (39%), pneumonia (7%) and postoperative bleeding requiring reoperation (23%).
Table 3
| Complication | n [%] |
|---|---|
| Operative mortality | 0 (0) |
| Mortality at 30 days | 2 [3] |
| Atrial fibrillation | 14 [24] |
| Pneumonia | 4 [7] |
| Prolonged air leak >7 days | 22 [38] |
| Respiratory failure | 1 [2] |
| Myocardial infarction | 2 [3] |
| Postoperative bleeding requiring reoperation | 13 [22] |
This series includes 19 patients who underwent adjuvant or neoadjuvant chemotherapy for NSCLC, with or without radiation therapy.
There were 42 patients in four-port, 56 patients in three-port and 302 patients in two-port group.
A PS analysis gained satisfactory balance among the three groups (Figure 1). Propensity-score adjusted differences among groups are presented in Table 4. Compared with two- and three-port group, patients in the four-port group had increased duration of chest tube (respectively difference and 95% CI are 1.493, 0.965; 2.053 and 1.246, 0.472; 2.002), increased postoperative length of stay (respectively difference and 95% CI are 2.564, 1.336; 3.952 and 2.205, 0.672; 3.740), increased postoperative pain only in comparison with two-ports (difference and 95% CI in VAS score 1.482, 0.909; 2.055). There were no significant differences in terms of demographic characteristics, histology, type of intervention, number of complications, operative time, number of lymph nodes retrieved and pStage between the three groups.
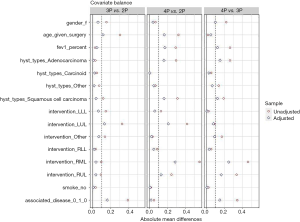
Table 4
| Outcomes considered | Groups comparison | Average difference | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| Time of surgical procedure in minutes | 3P vs. 2P | 3.428 | −11.953 | 19.313 |
| 4P vs. 2P | −9.623 | −20.248 | 2.007 | |
| 4P vs. 3P | −13.372 | −31.049 | 3.103 | |
| Number of retrieved lymph nodes | 3P vs. 2P | 0.664 | −1.551 | 2.899 |
| 4P vs. 2P | −1.103 | −2.464 | 0.296 | |
| 4P vs. 3P | −1.719 | −4.434 | 0.659 | |
| Occurrence of complications | 3P vs. 2P | −0.052 | −0.142 | 0.084 |
| 4P vs. 2P | −0.018 | −0.14 | 0.197 | |
| 4P vs. 3P | 0.036 | −0.139 | 0.268 | |
| Days of chest tube duration | 3P vs. 2P | 0.282 | −0.319 | 0.885 |
| 4P vs. 2P | 1.493 | 0.965 | 2.053 | |
| 4P vs. 3P | 1.246 | 0.472 | 2.002 | |
| Days of hospital stay | 3P vs. 2P | 0.395 | −0.492 | 1.358 |
| 4P vs. 2P | 2.564 | 1.336 | 3.952 | |
| 4P vs. 3P | 2.205 | 0.672 | 3.740 | |
| Pain | 3P vs. 2P | 1.052 | 0.822 | 1.279 |
| 4P vs. 2P | 1.482 | 0.909 | 2.055 | |
| 4P vs. 3P | 0.43 | -0.224 | 1.052 | |
| pStage | ||||
| IA | 3P vs. 2P | −0.041 | −0.211 | 0.12 |
| 4P vs. 2P | −0.059 | −0.136 | 0.064 | |
| 4P vs. 3P | 0.067 | −0.039 | 0.207 | |
| IB | 3P vs. 2P | 0.011 | −0.05 | 0.12 |
| 4P vs. 2P | 0.023 | −0.068 | 0.15 | |
| 4P vs. 3P | −0.266 | −0.66 | 0.17 | |
| IIA | 3P vs. 2P | −0.071 | −0.176 | 0.098 |
| 4P vs. 2P | −0.038 | −0.139 | 0.139 | |
| 4P vs. 3P | 0.426 | −0.072 | 0.974 | |
| IIB | 3P vs. 2P | −0.051 | −0.132 | 0.102 |
| 4P vs. 2P | −0.235 | −0.685 | 0.264 | |
| 4P vs. 3P | −0.012 | −0.157 | 0.183 | |
| IIIA | 3P vs. 2P | −0.108 | −0.276 | 0.087 |
| 4P vs. 2P | 0.427 | −0.13 | 0.986 | |
| 4P vs. 3P | −0.073 | −0.22 | 0.11 |
Discussion
To date, there is not a standardized approach to VATS lobectomy for non-small cell lung cancer (31). Despite most centres use a three-ports approach (two ports and an additional utility incision) (4), reducing the number and the length of incisions has become an irresistible trend (32,33). For instance, Gonzalez-Rivas developed a uniportal approach for VATS lobectomy (34).
We share the same route of VATS learning curve as Gonzalez-Rivas group. From May 2012 till December 2017, we perform 400 VATS lobectomies and in this review we would compare the outcome of each ‘phase’ of our experience (42 cases of four-ports, 56 cases of three ports and 302 cases of biportal VATS lobectomies) (20).
During the last 5 years of practicing VATS lobectomy, we have understood that to gain knacks of biportal VATS is important to apply two important changes of perspectives to the traditional three-port approach (35).
The first one is to achieve a satisfactory exposure even in the case of single utility incision (multiple instrument insertion) (36).
We observed that plastic wound protector/retractor (e.g., Alexis®) and rigid trocar would not be necessary because ‘naked’ utility incision does not obstacle the placement of the instrument or the working space (37).
Minimize the number of instruments simultaneously used seems to be very useful. In our opinion using an electronic hook (‘pick technique’) is useful to peel the mediastinal pleura and tissues surrounding hilar structures (38).
The right-angle clamp with the thread used for looping vessels sometimes needs of another curved forceps to fetch the thread. Alternatively, we use a silk thread with a circle at the tip fetched by a right angle clamp in order to directly loop the vessels.
By using just only electric hook and ultrasonic scalpel we perform a ‘no grasping’ technique to dissect lymph nodes. So, for resection of lymph node stations 7, 8, R2, R4, L5 and L6 we would rather en bloc resection because it is simpler and less bloody. Routinely, two instruments are enough for exposure and dissection, sometimes three are needed. With this intent the inferior and the upper part of the utility incision is needed to belong the different instruments (39).
Also rotate the operating table to different body postures could facilitate the exposure. Then, each procedure could beneficiate by table rotation: hilar vascular dissection (posterior rotation), lymph node dissection (anterior rotation), paratracheal (head up) and subcarinal (head down) (40).
A surgical multiple axis view allowing different orientation is mandatory. So, we prefer a 30° 10 mm high definition video thoracoscope that can also offer a good zoom effect. To zoom in on specific anatomical structures is necessary for precise surgical manipulation avoiding in this way extensive exposition (41).
Based on our experience all operations started with the pulmonary ligament section which is prolonged up to posterior mediastinal pleura until turn around underneath the arch of the azygos vein or aorta at the top. Then, lymph node station 9, 8 and 7 are resected.
The second change that we have to take in account when we approach biportal VATS is how to introduce staplers without the third posterior port conveniently. Our tips to solve this problem are: change the position of the endostapler and thoracoscope depending on the hilar structure to be resected during upper and middle lobectomies; proper execution camera and utility port; using guidance before triggering; open the tunica adventitia of vessels before insertion and stapler; clamping with a vascular clip or direct cut with an ultrasonic scalpel is more convenient and economical for smaller vessels (42). We prefer a reticulating endoscopic linear cutter such as Echelon powered® (Johnson & Johnson, USA) or Tristaple or Signia (Covidien, USA).
Usually we perform a more anterior utility incision and more posterior camera port in order to avoid collisions between the endostapler and the other instruments. Finally, a simultaneous dissection of N1 lymph node allows to free the pulmonary vein with a sufficient length.
Instrumentation with both proximal and distal articulation (modern staplers, high definition 30° cameras and energy devices) seems to be more fitted for biportal procedures. So, thanks to this instrumentation the cross hand technique is often used (19,43,44).
According to Kwhanmein Kim (45) we use the anterior approach to the subcarinal area after left upper lobectomy to perform lymph node dissection just only in certain cases. The posterior approach is usually performed for left-sided stations 5–12 even if the average lymph nodes harvested are less than right side because this is a taxing and time-consuming manoeuvre (46).
For right sided operations we dissect stations 2, 3, 4, 7–12. We preserve the azygos vein because we believe that azygos vein could help us especially during dissection of the lymph node located beneath the bifurcation between azygos vein and vena cava which is critical for the excision of R2 and 4 stations.
Two-ports technique can be very difficult in the case of obese patients, in whom a three-port access is preferred (47).
At the same time, VATS pneumonectomy needs a third port for safe stapling of pulmonary artery and main bronchus.
Limitations of the study
The main limitation of the study is related to its relatively small sample size, which is however compatible with being a single centre study. In addition, the non-randomized and retrospective design of the study may induce several biases in comparing the surgical approaches under study. Nevertheless, to avoid such limitation, state-of-the-art PS analysis has been used to overcome such limitations. Indeed, the PS matching weighting approach used to control for potential confounders might have had made three populations less susceptible to selection biases and thus more comparable.
For what concerns the potential biases due to the “learning curve” of surgeons involved in the study, this has not been considered due to lack of reliable information in the retrospective records. The issues, which is becoming increasingly prevalent in medical literature deserves further and specific attention.
Conclusions
According to our experience above described we can affirm that the pros of biportal VATS are different and very important such as decreased of duration of chest tube and decreased pain.
Acknowledgments
We are grateful to Mr Michael O’Flaherty for the language revision.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Crisci and Luca Bertolaccini) for the series “Surgical Approaches to VATS Lobectomy: Meet the Experts” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.08.05). The series “Surgical Approaches to VATS Lobectomy: Meet the Experts” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Before the submission to the Editor our Institutional review board approved the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roviaro G, Varoli F, Rebuffat C, et al. Major pulmonary resections: pneumonectomies and lobectomies. Ann Thorac Surg 1993;56:779-83. [Crossref] [PubMed]
- Hermansson U, Konstantinov IE, Arén C. Video-assisted thoracic surgery (VATS) lobectomy: the initial Swedish experience. Semin Thorac Cardiovasc Surg 1998;10:285-90. [Crossref] [PubMed]
- McKenna RJ Jr, Fischel RJ, Wolf R, et al. Video-assisted thoracic surgery (VATS) lobectomy for bronchogenic carcinoma. Semin Thorac Cardiovasc Surg 1998;10:321-5. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favourable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70. [Crossref] [PubMed]
- Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81; discussion 981-3. [Crossref] [PubMed]
- Yamashita JI, Kurusu Y, Fujino N, et al. Detection of circulating tumor cells in patients with non-small cell lung cancer undergoing lobectomy by video-assisted thoracic surgery: a potential hazard for intraoperative hematogenous tumor cell dissemination. J Thorac Cardiovasc Surg 2000;119:899-905. [Crossref] [PubMed]
- Kawachi R, Tsukada H, Nakazato Y, et al. Morbidity in video-assisted thoracoscopic lobectomy for clinical stage I non-small cell lung cancer: is VATS lobectomy really safe? Thorac Cardiovasc Surg 2009;57:156-9. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and metaanalysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small -cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 2012;1:16-23. [PubMed]
- Kim K, Kim HK, Park JS, et al. Video-assisted thoracic surgery lobectomy: single institutional experience with 704 cases. Ann Thorac Surg 2010;89:S2118-S2122. [Crossref] [PubMed]
- Mahtabifard A, McKenna RJ Jr. Video-assisted thoracic surgery for wedge resection, lobectomy, and pneumonectomy. In: Shields TW, LoCicero J, Reed CE, et al. editors. General thoracic surgery. 7th edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2009:523-31.
- Shigemura N, Akashi A, Nakagiri T, et al. Complete versus assisted thoracoscopic approach: a prospective randomized trial comparing a variety of video-assisted thoracoscopic lobectomy techniques. Surg Endosc 2004;18:1492-7. [Crossref] [PubMed]
- He J, Shao W, Cao C, et al. Long-term outcome and cost-effectiveness of complete versus assisted video-assisted thoracic surgery for non-small cell lung cancer. J Surg Oncol 2011;104:162-8. [Crossref] [PubMed]
- Göttgens KW, Siebenga J, Belgers EH, et al. Early removal of the chest tube after complete video-assisted thoracoscopic lobectomies. Eur J Cardiothorac Surg 2011;39:575-8. [Crossref] [PubMed]
- Borro JM, Gonzalez D, Paradela M, et al. The two-incision approach for video-assisted thoracoscopic lobectomy: an initial experience. Eur J Cardiothorac Surg 2011;39:120-6. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D’Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multiinstitution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Gossot D, Girard P, Raynaud C, et al. Totally endoscopic major pulmonary resection for stage I bronchial carcinoma: initial results. Rev Mal Respir 2009;26:961-70. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Gonzalez D, de la Torre M, Paradela M, et al. Video-assisted thoracic surgery lobectomy: 3-year initial experience with 200 cases. Eur J Cardiothorac Surg 2011;40:e21-8. [Crossref] [PubMed]
- Chen FF, Zhang D, Wang YL, et al. Video-assisted thoracoscopic surgery lobectomy versus open lobectomy in patients with clinical stage non-small cell lung cancer: a meta-analysis. Eur J Surg Oncol 2013;39:957-63. [Crossref] [PubMed]
- Yang B, Zhao F, Zong Z, et al. Preferences for treatment of lobectomy in Chinese lung cancer patients: video-assisted thoracoscopic surgery or open thoracotomy? Patient Prefer Adherence 2014;8:1393-7. [Crossref] [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [Crossref] [PubMed]
- Desai H, Natt B, Kim S, et al. Decreased in-hospital mortality after lobectomy using video-assisted thoracoscopic surgery compared with open thoracotomy. Ann Am Thorac Soc 2017;14:262-6. [PubMed]
- Bendixen M, Jorgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Salati M, Brunelli A, Rocco G. Uniportal video-assisted thoracic surgery for diagnosis and treatment of intrathoracic conditions. Thorac Surg Clin 2008;18:305-10. vii. [Crossref] [PubMed]
- Sihoe AD, Au SS, Cheung ML, et al. Incidence of chest wall paresthesia after video-assisted thoracic surgery for primary spontaneous pneumothorax. Eur J Cardiothorac Surg 2004;25:1054-8. [Crossref] [PubMed]
- Wildgaard K, Ringsted TK, Hansen HJ, et al. Quantitative sensory testing of persistent pain after video-assisted thoracic surgery lobectomy. Br J Anaesth 2012;108:126-33. [Crossref] [PubMed]
- Jiao P, Wu QJ, Sun YG, et al. Comparative study of three-dimensional versus two-dimensional video-assisted thoracoscopic two-port lobectomy. Thorac Cancer 2017;8:3-7. [Crossref] [PubMed]
- Huang W, Liu J, Liang W, et al. Outcome and Safety of Radical Resection in Non-Small Cell Lung Cancer Patients via Glasses-Free 3-Dimensional Video-Assisted Thoracoscope Versus 2-Dimensional Video-Assisted Thoracoscope. Surg Innov 2018;25:121-7. [Crossref] [PubMed]
- Ettinger DS, Bepler G, Bueno R, et al. Non-small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw 2006;4:548-82. [Crossref] [PubMed]
- Gonzalez-Rivas D. VATS lobectomy: surgical evolution from conventional VATS to uniportal approach. ScientificWorldJournal 2012;2012:780842 [Crossref] [PubMed]
- Yang R, Jiao W, Zhao Y, et al. Lymph node evaluation in totally thoracoscopic lobectomy with two-port for clinical early-stage non-small cell lung cancer: single-center experience of 1086 cases. Indian J Cancer 2015;52:e134-9. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Salati M, Rocco G. The uni-portal video-assisted thoracic surgery: achievements and potentials. J Thorac Dis. 2014;6:S618-S622. [PubMed]
- Wang GS, Wang Z, Wang J, et al. Uniportal complete video-assisted thoracoscopic lobectomy with systematic lymphadenectomy. J Thorac Dis 2014;6:1011-6. [PubMed]
- Kim HK, Choi YH. The feasibility of single-incision video-assisted thoracoscopic major pulmonary resection performed by surgeons experienced with a two-incision technique. Interact Cardiovasc Thorac Surg 2015;20:310-5. [Crossref] [PubMed]
- Hansen HJ, Petersen RH. Video-assisted thoracoscopic lobectomy using a standardized three-port anterior approach - The Copenhagen experience. Ann Cardiothorac Surg 2012;1:70-6. [PubMed]
- Wright GM. VATS lobectomy lymph node management. Ann Cardiothorac Surg 2012;1:51-5. [PubMed]
- Bedetti B, Scarci M, Gonzalez-Rivas D. Technical steps in single port video-assisted thoracoscopic surgery lobectomy. J Vis Surg 2016;2:45. [Crossref] [PubMed]
- Bertolaccini L, Rocco G, Viti A, et al. Geometrical characteristics of uniportal VATS. J Thorac Dis 2013;5:S214-S216. [PubMed]
- Chang JM, Kam KH, Yen YT, et al. From biportal to uniportal video-assisted thoracoscopic anatomical lung resection: A single-institute experience. Medicine (Baltimore) 2016;95:e5097 [Crossref] [PubMed]
- Jiao W, Zhao Y, Wang X, et al. Video-assisted thoracoscopic left upper lobe sleeve lobectomy combined with pulmonary arterioplasty via two-port approach. J Thorac Dis 2014;6:1813-5. [PubMed]
- Jiao W, Zhao Y, Huang T, et al. Two-port approach for fully thoracoscopic right upper lobe sleeve lobectomy. J Cardiothorac Surg 2013;8:99. [Crossref] [PubMed]
- Kim K. Video-assisted Thoracic Surgery Lobectomy. Korean J Thorac Cardiovasc Surg 2011;44:1-8. [Crossref] [PubMed]
- Di Rienzo G, Surrente C, Lopez C, et al. Tips and tricks in video-assisted thoracoscopic surgery lobectomy. Future Oncol 2016;12:35-8. [Crossref] [PubMed]
- Grossi W, Masullo G, Londero F, et al. Small incisions, major complications: video-assisted thoracoscopic surgery management of intraoperative complications. J Vis Surg 2018;4:12. [Crossref] [PubMed]
Cite this article as: Andriolo LG, Lopez C, Gregori D, Imbriglio G, Bottigliengo D, Surrente C, Larocca V, Di Rienzo G. Pros-cons debate about the role and evolution of biportal video-assisted thoracoscopic surgery. Shanghai Chest 2018;2:67.


