Results in robotic surgery for lung cancer
Introduction
Robotic surgery represents an advancement of minimally invasive surgical techniques, with augmented vision via a ten-magnified view, improved precision control via wristed instruments, and superior, seated ergonomics for the surgeon. The question remains whether these fundamental improvements translate to better outcomes for patients. Does the improved physician’s experience in a seated position with a high definitive view translate into better patient outcomes or greater financial windfall? Similar to a video-assisted thoracoscopic surgery (VATS) approach, robotic surgery, like all minimally invasive platforms, offers several clinical benefits, such as fewer overall complications, reduced pain, shorter length of stay, better postoperative pulmonary function, lower operative blood loss, and a lower 30-day mortality rate compared to open thoracotomy (1-6). Robotic surgery, however, comes at a higher initial cost. In this chapter, we review the surgical technique of robotic lobectomy for lung cancer and review some of the most recent data on clinical outcomes compared to other surgical approaches.
Initial evaluation
In order to accurately compare outcomes in cancer patients, patients must be carefully staged, using the same metrics. Many studies that compare patient outcomes are flawed because patients are not staged in the same way. We follow a standardized evaluation of patients with pulmonary nodules or masses that involves an algorithmic approach to preoperative studies, regardless of the anticipated platform or approach to be used. Initially, patients are often found to have a suspicious lesion on computed tomography (CT) scan, or present with biopsy-proven lung cancer. These patients then undergo a whole-body positron emission tomography-computed tomography (PET-CT) scan. Pulmonary reserve is measured with pulmonary function testing including diffusion capacity for carbon monoxide (DLCO) and spirometry. If indicated, mediastinal staging is achieved with endobronchial ultrasound-guided fine-needle aspiration biopsy (EBUS-FNA) or mediastinoscopy. These practices depend on the physician performing the procedure and institutional practice. Patients with a history of heart disease, or multiple cardiac risk factors, may warrant a cardiac stress-test. A brain MRI is obtained if there are concerns for metastatic disease or if the patient is experiencing neurologic symptoms.
One possible advantage to the robotic approach is that more patients can have a minimally invasive operation when a robotic platform is chosen. Although many expert VATS surgeons would disagree with this statement, it is probably true across a large population of surgeons. At present, there are no absolute contraindications to robotic surgery. In the past, some surgeons have suggested that tumor invasion of the vasculature, invasive T4 lesions, massive tumors (>10 cm), or Pancoast tumors, are relative contraindications for a robotic lobectomy, but we have performed safe and successful robotic operations in all of these scenarios. We have also performed sleeve resection of the main stem bronchus, both of the upper lobes and the middle lobe, chest wall resections, first rib resection for thoracic outlet syndrome, resections after neoadjuvant chemotherapy and/or radiation and/or immunotherapy, prior thoracotomy, and calcified or malignant hilar nodal disease. None of these diseases are contraindications for robotic-assisted lobectomy in the hands of experienced surgeons.
Equipment
Since the robotic platform has evolved so quickly and continues to do so, in order to ensure we are fairly and correctly comparing outcomes we need to ensure we document and understand the robotic systems used. To date, the Food and Drug Administration (FDA) has only approved the da Vinci robotic system from Intuitive Surgical (Intuitive Surgical Inc., Sunnyvale, CA, USA), but other computer-assisted platforms are currently in development. The design of the da Vinci system positions the operating surgeon at a console separate from the sterile field. Adjustable console components allow for an ergonomic, seated operating position. The patient is situated on a standard operating table which can be paired with the robotic or remain independent. The view of the operative field is available to the entire operating room (OR) team and surgical assistant on a viewing monitor on the vision cart, which can also be broadcast on OR screens or recorded. The current technology, the Xi version of the da Vinci robotic system, allows greater functionality than its previous generations including autonomous stapling and the use infrared fluorescence technology. The Xi also is more compatible with the OR, featuring an overhead mounting of the robotic arms that is driven over the patient, which is laser guided, and can be rotated to accommodate various patient positions or operative approaches. Compared to previous generations, the Xi has greater clearance of the robotic arms and has slimmer, longer arms (5 cm). The surgical team is also able to move the camera to any arm/port, which is often referred to as camera or port hopping. Although the older models such as the Si and S are unable to perform these functions, they remain safe and efficient for lung surgery. Our experience with the Si robot has spanned over 600 lobectomies with only a single 30- and 90-day mortality (4). A number of adjunctive technologies are integrated into the newer robotic systems (Si and Xi). Advanced EndoWrist autonomous staplers, vessel sealers, and energy instruments have been designed for greater functionality and efficiency. Infrared visualization technology via the robotic camera (Firefly) allows fluorescence of structures with the introduction of indocyanine green (ICG). During wedge resection or segmentectomy, we utilize ICG contrast using preoperative navigational bronchoscopy to localize subcentimeter lesions or ground-glass opacities that are difficult to palpate or visualize.
While the robot allows for greater control by the operating surgeon, an efficient and successful robotic operation is team dependent. A skilled bedside assistant is critical for fluid instrument exchange and maneuvers at the robot-patient interface. Clear communication between members of the surgical team is essential to a coordinated robotic operation. An optional second console is a particularly effective training tool, permitting a clear field of view for the trainee as well as fluid back-and-forth control of the robot instruments.
Patient positioning and port placement
In coordination with anesthesia, the patient is placed in lateral decubitus with the operative side exposed and the appropriate arm and thoracic supports are secured. We have previously described our “lean approach” to the OR: placement of the double lumen endotracheal tube in under one minute, the elimination of non-valued steps, and the elimination of useless items such as axillary rolls, arm boards, bean bags, arterial lines, central lines, epidurals, and type, screens, and cross-matching (7).
In general, for a right-sided case, the robotic ports insert above the ninth rib. For the left chest, we place our ports above the eighth rib. For an Xi robot, our we map out the ports in the following distribution: robotic arm 3 (5-mm port) is placed 1 to 2 cm from the spinous process of the vertebral body, robotic arm 2 (8 mm) is 10 cm medial to robotic arm 3, the camera port (we prefer the 12-mm) is 9 cm medial to robotic arm 2, and robotic arm 1 (12 mm) is placed right above the diaphragm anteriorly. The assistant port is triangulated between three other landmarks: the furthest anterior port, the camera, and as low in the chest to allow free movement of the instruments, just above the diaphragm. Our anecdotal experience is that a zero-degree camera reduces postoperative pain from decreased tension on the rib-space. We utilize a Conmed system (Conmed Edison, New Jersey, USA) via a 7-mm port to instill carbon dioxide into the chest, which serves to limit bleeding, and suppress the lung and lower the diaphragm, both increasing the operative field. Port placement is done safely under direct visualization and in coordination with each member of the team, including the surgeon, the surgical assistant, and anesthesia. Correct placement of ports is vital to a successful robotic operation.
Description of robotic lobectomy (Si robot)
After port placement and CO2 insufflation, the initial task is to explore the pleura to confirm the absence of metastatic lesions. The diaphragm is also evaluated. If metastatic deposits are found, they are biopsied and sent to pathology for intraoperative evaluation. Next, for a robotic lobectomy for non-small cell lung cancer, an aggressive thoracic lymphadenectomy is completed, including the N2 and N1 lymph nodes. An “aggressive” dissection means that we make a strong effort to clear out the entire lymph node package for each station. This method assures a high lymph node count for pathology and secures hemostasis. A complete lymphadenectomy assures accurate staging. Removal of the N1 lymph nodes also aids exposure of the pulmonary hilum, which clarifies structures in proceeding with lobectomy.
We prefer a posterior to anterior approach to the majority of lobectomies. The posterior approach begins with the removal of the N2 lymph nodes, unless a patient is to undergo a segmentectomy. In this case, we prefer to remove the N1 regional lymph nodes first and send them for frozen pathologic analysis. If positive for carcinoma and if the patient’s pulmonary function allows for lobectomy, a lobectomy is completed. If the patient cannot tolerate a lobectomy based on lung function, we do not send the regional N1 nodes, but proceed directly with segmentectomy. If a tissue diagnosis is needed prior to lobectomy and the lesion is amenable to a wedge resection, then we perform this first, send it for frozen section, and then remove the N2 and N1 lymph node stations.
Description of N2 lymph node dissection
Right side: the station 9 lymph nodes are exposed by division of the inferior pulmonary ligament, which also allows cranial retraction of the lower lobe. Next, the station 8 lymph nodes are removed with attention to avoid injury to the esophagus. Robotic arm 3, the most posterior arm, is used to retract the right lower lobe medially and anteriorly to remove lymph nodes from station 7. Robotic arm 3 is also used to retract the right upper lobe inferiorly during dissection of stations 2R and 4R. We begin dissection of these nodal stations medially along the superior vena cava and prefer to take the 4R and 2R lymph nodes en bloc clearing the space between the esophagus posteriorly and the azygos vein inferiorly. We then remove the lymph nodes from station 10, clearing the tissues beneath the azygos vein.
Left side: similar to the right side, the left lower lobe is retracted cranially and the inferior pulmonary ligament is divided. The lymph nodes at stations 8 and 9 are removed. Station 7 is exposed, lateral to the esophagus, between the tissue plane adjacent the inferior pulmonary vein and the lower lobe bronchus. Robotic arm 3 is used to move the left lower lobe medial and anterior. Lastly, robotic arm 3 curves around the left upper lobe and moves it down, permitting dissection of the 5 and 6 lymph node stations. The left recurrent laryngeal nerve can be injured during dissection of the aortopulmonary window. It is difficult to access the station 2L lymph node from the left side due to obstruction of the aortic arch. However, the 4L lymph node is frequently found and removed.
Right upper lobectomy
Recently we have championed a posterior approach to most right upper lobectomies and this has made the operation quicker and safer. All structures are taken form the back and the lung is never flipped or turned. Initially, as described above, the inferior pulmonary ligament is divided and the lymph nodes in the 9, 8 and then 7 stations are removed. The station 11 lymph nodes, located in the space adjacent the bronchus intermedius and the right lower lobe, are excised. A robotic stapler is then used to divide the posterior fissure. The initial vascular structure to be divided is the posterior-ascending branch of the pulmonary artery. Next we dissect the peribronchial lymph nodes and transect the bronchus to the right-upper lobe. The second vascular structure we take is another pulmonary artery branch, the anterior apical trunk, leaving the right upper lobe vein last. The lobe is completed with division of the fissure anteriorly.
Right middle lobectomy
The inferior pulmonary ligament is divided and the lymph nodes in the 9, 8 and then 7 stations are removed. The most posterior robotic arm is used to help visualize the pulmonary hilum anteriorly, with retraction of the right middle lobe, posterior and lateral in the chest. We then develop a plane between the tissues between the right upper and middle lobar veins. This dissection exposes the right middle lobe vein. We use a vascular robotic stapler to divide the right middle lobe vein. Often a vessel loop can be used to encircle the vein prior to stapling—a technique that can be used for all vascular structures. We then evaluate the fissure and determine if it complete or not. If incomplete, we divide it anteriorly. Tension or inadvertent transection of the segmental arteries to the right lower lobe may cause bleeding. The station 11 lymph nodes are removed from around the right middle lobe bronchus, clearing the tissues to allow stapling. Attention to the right middle lobe artery is required during this part of the surgery in order to avoid injury, as the two structures are in close approximation. We use serial staple firing to complete the fissure posteriorly to the level of the superior segment branch to the right lower lobe, which is identified and preserved. The segmental artery to the right middle lobe is then divided, however, attention must be given to a potential second lobar segmental artery. In order to avoid injury during stapling of the middle lobe pulmonary arteries, the stapler is passed posteriorly, so the tip isn’t striking other structures. Lastly, division of the fissure is completed, separating the right upper lobe from the right middle lobe.
Right lower lobectomy
The lymph nodes in the 9, 8 and then 7 stations are removed after division of the inferior pulmonary ligament. The station 11 lymph nodes between the right upper lobe and bronchus intermedius are dissected. Stapling of the posterior fissure is completed between the right upper and lower lobes. If the fissure is thick, then dissection is started anteriorly near the right middle lobe vein. The pulmonary artery is identified and a tunnel approach is used to stay on the pulmonary artery and then sequentially staple the fissure between the right middle lobe and lower lobe and then finally, the right lower lobe and upper lobe. The pulmonary artery is identified, specifically the superior segment artery. This artery, together with the right basilar pulmonary arterial branches, is divided, allowing the middle lobe bronchus to be easily seen. The inferior pulmonary vein is then divided. Lastly, the peribronchial lymph nodes are removed and the bronchus to the lower lobe is divided.
Left upper lobectomy
Again we favor a posterior approach. Similarly, the lymph node stations 9, 8, and 7 are taken as in the right chest. To access the lymph nodes station 10 and 11, we retract the lung to access the posterior hilum and dissect them from the ongoing pulmonary artery. With the lung in this position, the posterior segmental artery to the left upper lobe and the superior segmental artery to the left lower lobe are delineated. This exposes the fissure, which is subsequently stapled. Then the posterior segmental artery is taken and the lingular artery is divided. The left upper lobe vein is divided next by bringing the stapler from a posterior port. The left upper lobe bronchus is taken next if the anterior apical trunk cannot be seen. If this step is deemed unsafe, then the bronchus can be cut distally, while the remaining pulmonary artery branch is stapled and the bronchus is taken last. However, this is generally unnecessary and the anterior apical pulmonary trunk is usually taken last.
Left lower lobectomy
The inferior pulmonary ligament is divided and the lymph nodes in the 9, 8 and then 7 stations are removed. The station 10 and 11 lymph nodes are removed as described above. The fissure is exposed by dissection of the tissues adjacent the superior segmental and posterior segmental arteries. The fissure is stapled posterior to anterior. If the fissure is thick, then dissection is started anteriorly near the lingular vein. The pulmonary artery is identified and a tunnel approach is used to stay on the pulmonary artery and then sequentially staple the fissure between the left upper and lower lobes. The pulmonary artery is identified, specifically the superior segment artery, there are often two on the left side and one is posterior and can be easily missed. Unlike a right lower lobectomy, typically these branches are taken separately. The inferior pulmonary vein is divided. Peribronchial lymph nodes are removed and the left lower lobe bronchus is divided with a stapler, completing the lobectomy.
Long term results
During the past decade robotic thoracic surgeries have increased in number from 0.5% of lobectomies performed in 2008 to 20% in 2015. These numbers continue to rise, as more data is reported suggesting the benefits of a minimally invasive pulmonary resection with a robotic system (5,8). In many centers, case volume for open and VATS procedures are being replaced with robotic volume—this is particularly the case for high-volume hospitals and academic centers (9). As more surgeons move beyond their initial learning curves, the number of robotic cases will inevitably increase. As an alternative to both open surgery and VATS, the adoption of robotic thoracic surgery has, so far, outpaced that of VATS or single-port approaches.
Recent literature has shown outstanding short-term clinical outcomes in patients undergoing pulmonary resection for lung cancer. Metrics of surgical quality, such as lymph node resection, rate of R0 resection, conversion to open thoracotomy, readmission rate, and length of stay, have all shown improvement with a robotic approach verses open surgery (5,9-11). In our own experience, we have demonstrated a 30-day mortality rate of less than 1% (0.19%), a low 90-day mortality rate (0.57%), and major morbidity rate of 9.6% in patients undergoing robotic lobectomy. Other groups have published impressive outcomes data as well (as shown in Table 1) (9-11,16,18). Kent and colleagues have shown in a national database study of over 33,000 patients, that mortality after lobectomy can be lower using a robotic approach rather than an open thoracotomy (5). Equally, their data revealed a trend toward superior outcomes with the robotic versus VATS lobectomy. Uniquely, their study involved a wide geography, involving a variety of clinical settings, including academic and community hospitals, revealing that robotic surgery programs can be successful in diverse resource environments. In another study, Burt and colleagues revealed that robotic lobectomy could be completed safely in patients with marginal pulmonary function as evidenced by pulmonary function testing (6).
Table 1
| Year | N | Conversion rate | Morbidity | Perioperative mortality | Median LOS (day) | Notes |
|---|---|---|---|---|---|---|
| Cerfolio et al. (7) | 520 | 12% (first 100 cases), 3.3% (last 120 cases) | 50% (first 100 cases), 4.2% (last 120 cases) | 0.19% (30-d), 0.57% (90-d) | 3 | – |
| Yang et al. (12) | 172 | 9% | 26% | 0% | 4 | Equivalent OS and DFS at 5 yrs. to VATS |
| Veronesi et al. (8) | 54 | 13% | 20% | 0% | 4.5 | – |
| Gharagozloo et al. (13) | 100 | – | 21% | 3% | 4 | – |
| Echavarria et al. (14) | 208 | 9.6% | 40.4% | 1.44% (in hospital) | 5 | – |
| Louie et al. (10) | 1,220 | Not reported | No difference from VATS | 0.3 (in hospital), 0.6% (30-d) | 4 | 8.44% nodal upstaging |
| Toker et al. (15) | 102 | 4% | 24% | 2% (60-d) | 5 (mean) | 104 min (mean operative time) |
| Adams et al. (16) | 116 | 3.3% | No difference from VATS | 0% (30-d) | 4.7 (mean) | – |
| Melfi et al. (17) | 229 | 10.5% (first 69 cases), 5.6% (next 160 cases) | 22% and 15% | 1.4% and 0% | 4.4 and 3.8 (mean) | – |
Data included in citations. DFS, disease-free survival; LOS, length of stay; OS, overall survival; QOL, quality of life; VATS, video-assisted thoracoscopic surgery.
While comparison with open thoracotomy may show a clear advantage for the robotic, other data, however, has shown more limited advantages to a robotic system over current thoracoscopic techniques. In a nationwide study comparing robotic surgery with a VATS approach, Rajaram and colleagues, revealed no difference between robotic and thoracoscopic lobectomy in a number of important outcome measures including mortality, readmission, and rate of R0 resection (9). They also revealed that patients undergoing a robotic resection had fewer lymph nodes resected and fewer lymph node stations assessed. Overall, the data on lymph node resection is mixed and the reason for this fact may be that the completeness of resection is more dependent on the surgeons’ desire to perform a thorough lymph node dissection then the platform used (16).
There are substantial initial financial costs with starting a robotic program. Moreover, there are substantial operational costs, including maintenance costs of the hardware and expendable instruments and robotic training for the surgeon and bedside assistant, both of which require strong institutional cooperation and coordination (19). In addition, there is always a learning curve when starting something new during which quality can suffer and operating efficiency decreases. We define quality with the metric: value = quality/cost. Quality must be objective and assessed in monetary units.
Perhaps the best judge of value is the stage specific 5-year survival, not just short-term metrics, as we have been using for the past decade to judge the quality of our practices. Historically, the quality of an operation has been measured through the limited view of 30- and 90-day outcomes. These metrics have gained their ascendancy via insurance companies and hospitals. The absolute value of an expense extends throughout the duration of its result, or in other terms, its durability. Financial costs, such as those required of robotic systems, may provide an overall cost savings if the systems can provide durable quality outcomes. Durability in this regard is reflected in both recurrence and long-term patient survival. Unfortunately, there are limited reports that show these important metrics of value for pulmonary resection for lung cancer.
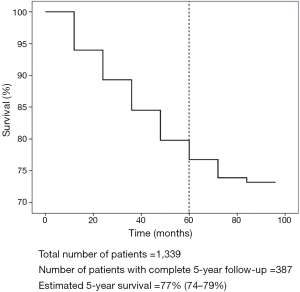
The largest report of robotic lobectomy, with the longest follow-up, was recently published by our group (as shown in Figure 1) (20). Our data reveal a stage specific survival for patients who have undergone an R0 resection for non-small cell carcinoma (83% stage IA, 77% stage IB, 68% for stage IIA, 70% for stage IIB, 62% for stage IIIA, and 31% for stage IIIB), that compares favorably with most every other large series that has reported stage-specific survival for VATS or thoracotomy (21-26) (as shown in Table 2). Additionally, our short-term clinical outcomes were promising, including mean operative time (136 minutes), blood loss (50 mL/case), median length of stay (3 days), and rate of overall morbidity (8%).
Table 2
| Type of operation | Thomas 2002 (22) | Walker 2003 (23) | Yildizeli 2007 (25) | Flores 2009 (26) | Lee 2013 (27) | Park 2012 (21) | Cerfolio 2018 (20) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VATS | Thoracotomy | VATS | Thoracotomy | VATS | Thoracotomy | VATS | Thoracotomy | Robotic | Robotic | |||||||
| Total number of patients | 110 | 405 | 158 | 218 | 398 | 343 | 208 | 208 | 325 | 1,339 | ||||||
| Median F/U in months | N/A | N/A | 38 (mean) | 79 | 28 | 28 | 36 | 36 | 27 | 30 | ||||||
| No. pts actually alive at 5 years (%) | 26 (24%) | 108 (27%) | N/A | N/A | 11 (3%) | 30 (9%) | 18 (9%) | 92 (44%) | 38 (12%) | 387 (29%) | ||||||
| % overall survival reported | 63% | 63% | N/A | 53% | 79% | 75% | 75% | 77% | 80% | 77% | ||||||
| Stage IA | Stage I | Stage I | Stage I | Stage I | ||||||||||||
| No. pts | 50 | 97 | 117 (48 IA, 70 IB) | 69 (21 IA, 48 IB) | 260 | 213 | 168 (120 IA, 48 IB) | 146 (105 IA, 41 IB) | 176 | 672 | ||||||
| No. pts actually alive at 5 years (%) | 11 (22%) | 31 (32%) | N/A | N/A | N/A | N/A | 16 (10%) | 75 (51%) | 27 (15%) | 234 (35%) | ||||||
| % survival reported | 65% | 80% | 78% | 57% | N/A | N/A | 79% | 84% | 91% | 83% | ||||||
| Stage IB | ||||||||||||||||
| No. pts | 60 | 308 | N/A | N/A | 69 | 62 | N/A | N/A | 72 | 281 | ||||||
| No. pts actually alive 5 years (%) | 15 (25%) | 77 (25%) | N/A | N/A | N/A | N/A | N/A | N/A | 6 (8%) | 71 (25%) | ||||||
| % survival reported | 61% | 58% | N/A | N/A | N/A | N/A | N/A | N/A | 88% | 77% | ||||||
| Stage IIA | Stage II | Stage II | Stage II | |||||||||||||
| No. pts | N/A | N/A | 33 (8 IIA, 25 IIB) | 86 (6 IIA, 80 IIB) | 19 | 17 | N/A | N/A | 56 (41 IIA, 15 IIB) | 118 | ||||||
| No. pts actually alive 5 years (%) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 5 (9%) | 29 (25%) | ||||||
| % survival reported | N/A | N/A | 51% | 70% | N/A | N/A | N/A | N/A | 49% | 68% | ||||||
| Stage IIB | ||||||||||||||||
| No. pts | N/A | N/A | N/A | N/A | 12 | 15 | N/A | N/A | N/A | 99 | ||||||
| No. pts actually alive 5 years (%) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 21 (21%) | ||||||
| % survival reported | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 70% | ||||||
| Stage IIIA | ||||||||||||||||
| No. pts | N/A | N/A | 8 | N/A | 29 | 21 | N/A | N/A | 21 | 143 | ||||||
| No. pts actually alive 5 years (%) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 26 (18%) | ||||||
| % survival reported | N/A | N/A | 29% | N/A | N/A | N/A | N/A | N/A | N/A | 62% | ||||||
No. pts, number of patients; VATS, video-assisted thoracoscopic surgery; N/A, not available.
Although our series shows favorable survival, there are other institutional series that reveal an alternative narrative. In a 2012 series by Park and colleagues, patients after robotic surgery that were followed over time, were found to have a lower survival rate when compared to our series (21). In this population, however, only 12% of patients were 5 years or greater from surgery compared to 29% of patients in our study. To be able to fully understand how a robotic approach can affect long-term outcome, larger data sets must be analyzed with longer follow-up. These studies are ongoing.
One possible explanation to the observed improved survival after minimally invasive operations compared to thoracotomy may be secondary to a lesser degree of an immune suppression. It has been theorized that a less invasive operation may produce lower cytokines (28) which may lead to a lower systemic rate of solid organ metastases. Another second theory of an increased survival rate of a robotic lobectomy is the improved staging of these patients, because of the ability to achieve a complete lymph node dissection (29,30). A more complete N1 and N2 lymph node resection likely results in improved staging and therefore, a greater number of patients likely undergo adjuvant chemotherapy, conferring a survival advantage in a number of these patients.
Another thoracic procedure gaining popularity on robotic systems is segmentectomy, which preserves lung parenchyma versus a lobectomy. Many minimally invasive surgeons believe the biggest advantage of the robot is for segmentectomy. Some data have revealed longer operating times for robotic segmentectomy when compared to lobectomy (219 vs. 175 minutes; P<0.01) (31). We have reported on 100 consecutive robotic segmentectomy and showed excellent perioperative results: 88 minutes median operative time, 7% conversion rate, 10% major postoperative complication rate, 0% 30- and 90-day mortality rates (32). To date, we have completed over 210 robotic segmentectomy with no 30- or 90-day mortalities. When to perform a segmentectomy rather than lobectomy remains an active area of research.
Summary
Robotic pulmonary resection has been demonstrated to be a safe and effective modality for lung cancer. Lobectomy and segmentectomy can be completed efficiently and with low conversion rates to thoracotomy. Perioperative morbidity and mortality is similar to thoracoscopic lobectomy and consistently better when compared to open surgery. Long-term oncologic outcomes for robotic lobectomy have shown promising results. In early 3–5 years follow-up studies, outcomes are similar or better than those demonstrated after VATS and open lobectomy. Reasons for this may be from offering an operation that has less inflammation and one that affords a better opportunity to obtain a complete lymph node dissection. More follow-up is needed. The technology employed in a robotic system provides subjective advantages to the surgeon, but these advantages must be quantified and measured. The true value of any operation should be carefully defined and measured from the vantage point of the patient first, but also from the perspective of the surgeon, trainees, as well as the from the hospital and the payer.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lorenzo Spaggiari and Domenico Galetta) for the series “Minimally Invasive Thoracic Oncological Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: The series “Minimally Invasive Thoracic Oncological Surgery” was commissioned by the editorial office without any funding or sponsorship. RJC discloses relations with Intuitive, Medtronic, Ethicon, KCL, Google, TransEnteric, Pinnacle, Myriad, Bovie, Covidien, Neomemd/BARD, Novartis and president of ROLO-7 consulting firm. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cao C, Manganas C, Ang SC, et al. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann Cardiothorac Surg 2012;1:3-10. [PubMed]
- Nakamura H. Systematic review of published studies on safety and efficacy of thoracoscopic and robot-assisted lobectomy for lung cancer. Ann Thorac Cardiovasc Surg 2014;20:93-8. [Crossref] [PubMed]
- Rinieri P, Peillon C, Salaün M, et al. Perioperative outcomes of video- and robot-assisted segmentectomies. Asian Cardiovasc Thorac Ann 2016;24:145-51. [Crossref] [PubMed]
- Bryant AS, Rudemiller K, Cerfolio RJ. The 30- versus 90-day operative mortality after pulmonary resection. Ann Thorac Surg 2010;89:1717-22; discussion 1722-3.
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Burt BM, Kosinski AS, Shrager JB, et al. Thoracoscopic lobectomy is associated with acceptable morbidity and mortality in patients with predicted postoperative forced expiratory volume in 1 second or diffusing capacity for carbon monoxide less than 40% of normal. J Thorac Cardiovasc Surg 2014;148:19-28, discussion 28-29.e1.
- Cerfolio RJ, Steenwyk BL, Watson C, et al. Decreasing the Preincision Time for Pulmonary Lobectomy: The Process of Lean and Value Stream Mapping. Ann Thorac Surg 2016;101:1110-5. [Crossref] [PubMed]
- Veronesi G, Cerfolio R, Cingolani R, et al. Report on First International Workshop on Robotic Surgery in Thoracic Oncology. Front Oncol 2016;6:214. [Crossref] [PubMed]
- Rajaram R, Mohanty S, Bentrem DJ, et al. Nationwide Assessment of Robotic Lobectomy for Non-Small Cell Lung Cancer. Ann Thorac Surg 2017;103:1092-100. [Crossref] [PubMed]
- Louie BE, Wilson JL, Kim S, et al. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using The Society of Thoracic Surgeons Database. Ann Thorac Surg 2016;102:917-924. [Crossref] [PubMed]
- Farivar AS, Cerfolio RJ, Vallières E, et al. Comparing robotic lung resection with thoracotomy and video-assisted thoracoscopic surgery cases entered into the Society of Thoracic Surgeons database. Innovations (Phila) 2014;9:10-5. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. [Crossref] [PubMed]
- Echavarria MF, Cheng A, Velez F, et al. Comparison of Peri-Operative Outcomes After Robotic-Assisted Video-Thoracoscopic Lobectomies Versus Segmentectomies. J Thorac Oncol 2016;11:S190-S191. [Crossref] [PubMed]
- Toker A, Ozyurthkan MO, Kaba E, et al. Robotic anatomic lung resections: the initial experience and description of learning in 102 cases. Surg Endosc 2016;30:676-83. [Crossref] [PubMed]
- Adams RD, Bolton WD, Stephenson JE, et al. Initial multicenter community robotic lobectomy experience: comparisons to a national database. Ann Thorac Surg 2014;97:1893-8; discussion 1899-900.
- Melfi FM, Fanucchi O, Davini F, et al. Robotic lobectomy for lung cancer: evolution in technique and technology. Eur J Cardiothorac Surg 2014;46:626-30. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Deen SA, Wilson JL, Wilshire CL, et al. Defining the cost of care for lobectomy and segmentectomy: a comparison of open, video-assisted thoracoscopic, and robotic approaches. Ann Thorac Surg 2014;97:1000-7. [Crossref] [PubMed]
- Cerfolio RJ, Ghanim AF, Dylewski M, et al. The long-term survival of robotic lobectomy for non-small cell lung cancer: A multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. [Crossref] [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Thomas P, Doddoli C, Yena S, et al. VATS is an adequate oncological operation for stage I non-small cell lung cancer. Eur J Cardiothorac Surg 2002;21:1094-9. [Crossref] [PubMed]
- Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397-402. [Crossref] [PubMed]
- Roviaro G, Varoli F, Vergani C, et al. Long-term survival after videothoracoscopic lobectomy for stage I lung cancer. Chest 2004;126:725-32. [Crossref] [PubMed]
- Yildizeli B, Fadel E, Mussot S, et al. Morbidity, mortality, and long-term survival after sleeve lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;31:95-102. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013;96:951-60. [Crossref] [PubMed]
- Flores RM, Ihekweazu UN, Rizk N, et al. Patterns of recurrence and incidence of second primary tumors after lobectomy by means of video-assisted thoracoscopic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2011;141:59-64. [Crossref] [PubMed]
- Kanagarajah P, Torres A, Bacigalupo A, et al. Robotic Video-Assisted Thoracoscopic Lung Resection: Comparison of Outcomes and Learning Curve from a Single Surgeon in a Tertiary Care Center. Am J Respir Crit Care Med 2016;193:A6022.
- Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901-6; discussion 1906-7.
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Cerfolio RJ, Watson C, Minnich DJ, et al. One Hundred Planned Robotic Segmentectomies: Early Results, Technical Details, and Preferred Port Placement. Ann Thorac Surg 2016;101:1089-95; Discussion 1095-6. [Crossref] [PubMed]
Cite this article as: Geraci TC, Cerfolio RJ. Results in robotic surgery for lung cancer. Shanghai Chest 2018;2:70.