
What are the implications of the revised N staging system for surgery?
Past and current lymph node staging for mesothelioma
Malignant pleural mesothelioma (MPM) is an intrathoracic malignancy with unique features. MPM has a poor prognosis and an average survival of 12–18 months despite treatment (1,2). One of the unique features of this malignancy is the widespread development of nodules inside the pleural cavity, with a tendency to form tumor islands over the diaphragm and the costophrenic sulcus mainly due to gravity. Diffuse nodules inside the pleural cavity leads to a chaotic and irregular lymph node metastasis pattern which involves the entire chest cavity.
Metastasis to hilar and intrapulmonary lymph nodes was observed in MPM in the initial studies. In a 1988 report from the Canadian Tumor Reference Center, in 77 autopsy cases with MPM, 34 had metastasis to bronchopulmonary, hilar or mediastinal lymph nodes (3). In surgically managed 157 patients, mediastinal lymph node metastasis was found in 52% of the patients (4).
Lymph node staging in 1995 International staging system for MPM was exactly the same as Lung Cancer lymph node staging (Table 1) (5). In the recent recommendations for N staging, the unique intrathoracic involvement sites (internal mammary, peridiaphragmatic, pericardial fat pad and intercostal lymph nodes) in MPM were stated for the first time in the N1 category. The current recommendations decrease lymph node groupings from 4 to 3 and are shown in Table 2 (6).
Table 1
| Regional lymph nodes (N) | Definition |
|---|---|
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastases |
| N1 | Metastases in the ipsilateral bronchopulmonary or hilar lymph nodes |
| N2 | Metastases in the subcarinal or ipsilateral mediastinal lymph nodes, including the ipsilateral internal mammary nodes |
| N3 | Metastases in the contralateral mediastinal, contralateral internal mammary, ipsilateral, or contralateral supraclavicular lymph node |
Table 2
| Regional lymph nodes (N) | Definition |
|---|---|
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastases |
| N1 | Metastases in the ipsilateral bronchopulmonary, hilar, or mediastinal (including the internal mammary, peridiaphragmatic, pericardial fat pad, or intercostal lymph nodes) lymph nodes |
| N2 | Metastases in the contralateral bronchopulmonary, hilar, or mediastinal lymph nodes or ipsilateral or contralateral supraclavicular lymph nodes |
AJCC/UICC, American Joint Committee on Cancer/Union for International Cancer Control.
Besides the international efforts, several centers with experience in MPM surgery and treatment proposed more simplified lymph node staging systems in MPM. These were similar to the initial staging system, as intrapleural lymph nodes were categorized as N1 where as extrapleural lymph nodes were categorized as N2. In a recent study involving 529 patients with epithelioid MPM who underwent extrapleural pneumonectomy (EPP), lymph node staging showed separate survival curves with worsening survival from N0 to N3. Further analysis of patients with both N1 and N2 involvement showed that these patients had a survival worse than patients with only N2 station involvement (3).
Pattern of lymph node involvement in mesothelioma
Pleural fluid is mainly absorbed through the lymphatics in the parietal pleura. Limited absorption occurs through visceral pleura. Animal studies were performed using near infrared fluorescent lymph tracer injected to the pleural space of rats and pigs. It was noted that lymphatic drainage of both pleural cavities as a sentinel lymph node were to the highest mediastinal lymph node station (station 1 as described in Lung Cancer lymph node map) initially (7). However this was followed by lymphatic spreading to other intrathoracic and extrathoracic lymph nodes (8).
In a 2006 report by Edwards et al., out of 92 patients who underwent EPP, 35 had N2 involvement (9). In those patients, 17 had positive lymph nodes inaccessible to mediastinoscopy. The sites were paraesophageal (n=7), pulmonary ligament (n=1), internal mammary (n=2), pericardial (n=3) and peridiaphragmatic (n=3). Similar results were reported in 53 patients. Diaphragmatic and internal mammary lymph nodes were the common sites of involvement in N2 positive patients (10). Another study was presented at the World Lung Cancer Conference by Friedberg showing involvement of posterior intercostal lymph nodes as a poor prognostic factor and also presence of posterior intercostal lymph node involvement in almost half of 48 patients (11).
Computed tomography (CT) analysis of 750 patients (91 with MPM) for different clinical purposes showed that pathologic size (>10 mm diameter) cardiophrenic and extrapleural lymph nodes were present in 26 and 68% of the patients with MPM which were significantly more frequent than other patients (12).
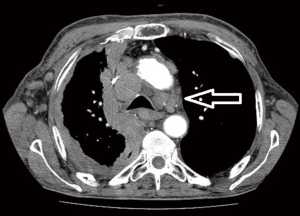
Computed tomographic views of internal mammary, intercostal, peridiaphragmatic and pericardial lymph nodes are shown in Figures 1-4.
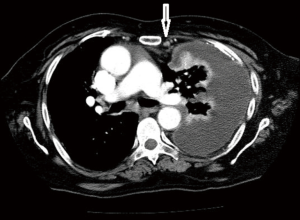
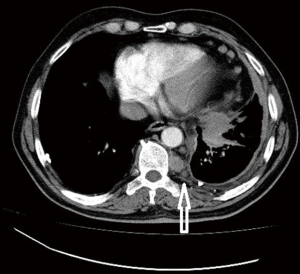
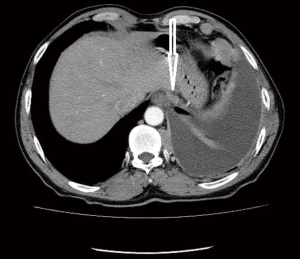
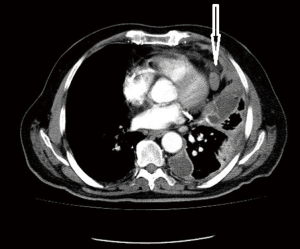
The initial report showing poor outcomes in patients with extrapleural and mediastinal lymph node involvement was published in 1993 (13). The poor prognostic impact of extrapleural lymph node involvement resulted in several studies analyzing the outcomes of pretreatment mediastinal staging techniques for MPM.
In one of the earliest reports, cervical mediastinoscopy was found to be superior to CT staging of mediastinal lymph nodes in MPM (14).
A report from Egypt showed that 6 of 14 patients with positive mediastinal lymph nodes also had N1 involvement (10). In one of the largest reports (529 patients) by Sugarbaker, 136 patients (26%) had concurrent N1 and N2 involvement, whereas only 45 (9%) had isolated N2 involvement (3). Additionally, de Perrot and colleagues showed a negative predictive value of only 68% for mediastinoscopy in the preoperative staging of MPM (15). Despite this low negative predictive value for mediastinoscopy, Pilling and colleagues advocated for pretreatment mediastinoscopy, as extrapleural lymph node involvement lead to a dismal median survival of 4.4 months, whereas those without lymph node involvement had a median survival of 16.4 months (16). In the same study, 15 of 17 patients with extrapleural lymph node involvement could have been diagnosed preoperatively with cervical mediastinoscopy. Thus the authors recommended a cervical mediastinoscopy for all MPM patients who would undergo a surgical treatment (16).
Transesophageal endoscopic ultrasound guided fine needle aspiration was also found to be reliable in mediastinal lymph node staging of MPM (17). In 21 patients, 4 were found to have mediastinal lymph node metastasis with endoscopic ultrasound-fine needle aspiration (EUS-FNA) and in remaining 17 patients, only 1 had a positive lymph node following surgical resection through thoracotomy. Sensitivity and specificity of EUS FNA were found to be 80% and 100% respectively. EUS FNA was also reported in 6 patients with MPM, of which 8 sites were sampled, 4 subcarinal, 3 aortopulmonary window and 1 paraesophageal. 2 were found to be positive for tumor and 2 were missed due to technical errors (18). There are other reports of patients that can be diagnosed with plain transbronchial needle aspiration biopsy using a 21-gauge needle for subcarinal and paratracheal lymph nodes and subsequent cytologic and immunohistochemical analysis or under endobronchial ultrasound (EBUS) guidance (19,20).
Surgical implications of the revised lymph node staging system
IASLC Mesothelioma Staging Project was an important collaboration with contributions from 29 centers around the world. Data of 3,519 patients were submitted with 1,566 patients entered prospectively. In the final analysis, 1,322 patients had clinical N data and 851 patients had pathologic N data (6). When patients were staged based on the 7th edition of AJCC, clinical N stages were similar to each other with survivals ranging from 19 to 14.5 months between N0 and N3 stages. However in the pathologic staging N1 and N2 had a median survival of 16.9 and 17.4 months whereas pathologic N0 patients had a median survival of 24 months. Additionally single or multiple station involvement, involvement of only N1, only N2 or concurrent involvement of both stations resulted in similar survival rates.
In a recent multicentric retrospective study from France including 99 patients who underwent EPP showed that lymph node involvement and also ratio of metastatic lymph nodes (>13%) were prognostic (21). The site or total number of lymph nodes involved were not prognostic. The median survival was almost halved (12.7 versus 22.4 months) in case of lymph node involvement and it was significantly shortened (11.7 versus 19.9 months) if ratio of metastatic lymph nodes to total number of lymph nodes was >13%.
There are three main issues with all of these studies and the newly proposed lymph node staging system in MPM.
As a first issue, in the IASLC Mesothelioma Project only 22% of the tumors in 1,029 patients had clinically involved lymph nodes, however following surgery 321 of 851 (38%) had lymph node metastasis which shows significant deficiencies in preoperative lymph node staging. In this database only 56% of the clinically staged patients had PET scan and 6% underwent a mediastinoscopy (3). The preoperative invasive staging is inadequate and almost 40% are understaged in clinical N0 patients and 30% in N1 patients. It is recommended to use invasive mediastinal staging with cervical mediastinoscopy, EBUS or EUS, however it is also apparent that pericardial and internal mammary lymph nodes are inaccessible even with these methods. Invasive preoperative mediastinal staging is important to stratify patients for appropriate treatment, because we currently have more effective treatments that can be used in a neoadjuvant protocol. In the randomized study using three chemotherapeutic agents (Bevacizumab, Pemetrexed and Cisplatin), 18.8 months median survival was observed with a 50% response rate (1).
Pathologic staging of lymph nodes are only obtained with invasive staging or during surgical resection, however the standards of lymphadenectomy or lymph node sampling in MPM surgery are not well described. Surgical technique has an impact on lymph node staging. In patients who undergo extended pleurectomy decortication (PD) or EPP, mediastinal staging is more thoroughly performed, whereas in case of a partial PD lymph node staging is limited which leads to a bias in survival outcomes (22). Our study addressing transition of surgical technique from EPP predominant to a PD predominant practice showed that our intraoperative lymph node staging rate dropped from 70% to 64%, but this did not translate to any survival disadvantage. When significant amount of gross disease is left behind, especially in the mediastinum, mediastinal lymph node staging is obviously hampered. The technique (sampling versus dissection) and principles of lymphadenectomy or sampling (which stations and how many lymph nodes) is not standardized in MPM surgery. The number and sites of potential lymph node involvement in MPM is much higher and widespread than lung cancer. The current staging system does not address this issue.
Third issue is the heterogeneity of survival in patients with lymph node involvement. Tumor thickness was proposed as a predictor of mediastinal lymph node involvement and survival in 65 patients who underwent radiation treatment and surgery (23). These patients had very thick tumors from 2.4 to 21 cm. Hazard ratio was 2.1, but 95% confidence interval was wide (1.07 to 4.33). In our study in 196 patients with a median tumor thickness of 2.1 cm, there was no cut off point for tumor thickness, presence of thickening was associated with poor survival (24). Almost 10% of the patients with bronchopulmonary or ipsilateral mediastinal and extrapleural lymph node involvement survive more than 5 years. On the other hand patients without lymph node involvement have a 5-year survival of 12–18%. Thus the difference is not very high. Based on the newly proposed staging system even if the patient has an ipsilateral mediastinal lymph node metastasis, he/she can be offered multimodality treatment including surgery that would lead to a median survival of 17 to 21 months. Contralateral lymph node involvement appears to be an absolute contraindication for surgery, as it has a very poor prognosis with no 5-year survivors (Figure 5).
In conclusion, the revised N staging system emphasizes the importance of preoperative invasive mediastinal staging, broadens surgical indications without dramatic improvements in survival. It shows that addition of surgery leads to a 5-year survival rate of 10% even in case of ipsilateral mediastinal, intrapulmonary or extrapulmonary lymph node metastasis. It appears that if invasive mediastinal staging is adopted, neoadjuvant treatment options will be more frequently utilized in MPM.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (David Waller and Annabel Sharkey) for the series “Mesothelioma Surgery” published in Shanghai Chest. The article did not undergo external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.09.02). The series “Mesothelioma Surgery” was commissioned by the editorial office without any funding or sponsorship. Dr. Batirel reports personal fees from JOHNSON AND JOHNSON, outside the submitted work. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14. [Crossref] [PubMed]
- Sugarbaker DJ, Richards WG, Bueno R. Extrapleural pneumonectomy in the treatment of epithelioid malignant pleural mesothelioma: novel prognostic implications of combined N1 and N2 nodal involvement based on experience in 529 patients. Ann Surg 2014;260:577-80; discussion 580-2. [Crossref] [PubMed]
- Huncharek M, Smith K. Extrathoracic lymph node metastases in malignant pleural mesothelioma. Chest 1988;93:443-4. [Crossref] [PubMed]
- Rusch VW, Venkatraman ES. Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann Thorac Surg 1999;68:1799-804. [Crossref] [PubMed]
- Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest 1995;108:1122-8. [Crossref] [PubMed]
- Rice D, Chansky K, Nowak A, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the N Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2100-11.
- Parungo CP, Ohnishi S, De Grand AM, et al. In vivo optical imaging of pleural space drainage to lymph nodes of prognostic significance. Ann Surg Oncol 2004;11:1085-92. [Crossref] [PubMed]
- Parungo CP, Colson YL, Kim SW, et al. Sentinel lymph node mapping of the pleural space. Chest 2005;127:1799-804. [Crossref] [PubMed]
- Edwards JG, Stewart DJ, Martin-Ucar A, et al. The pattern of lymph node involvement influences outcome after extrapleural pneumonectomy for malignant mesothelioma. J Thorac Cardiovasc Surg 2006;131:981-7. [Crossref] [PubMed]
- Abdel Rahman AR, Gaafar RM, Baki HA, et al. Prevalence and pattern of lymph node metastasis in malignant pleural mesothelioma. Ann Thorac Surg 2008;86:391-5. [Crossref] [PubMed]
- Friedberg J. Posterior intercostal lymph nodes – First report of a new independent prognostic factor for malignant pleural mesothelioma. (MO09-12) World Conference on Lung Cancer, Oct 27-30, 2013, Sydney, Australia.
- Feragalli B, Mantini C, Civitareale N, et al. Extrapleural and cardiophrenic lymph nodes: prevalence, clinical significance and diagnostic value. Radiol Med 2014;119:20-6. [Crossref] [PubMed]
- Sugarbaker DJ, Strauss GM, Lynch TJ, et al. Node status has prognostic significance in the multimodality therapy of diffuse, malignant mesothelioma. J Clin Oncol 1993;11:1172-8. [Crossref] [PubMed]
- Schouwink JH, Kool LS, Rutgers EJ, et al. The value of chest computer tomography and cervical mediastinoscopy in the preoperative assessment of patients with malignant pleural mesothelioma. Ann Thorac Surg 2003;75:1715-8; discussion 1718-9.
- de Perrot M, Uy K, Anraku M, et al. Impact of lymph node metastasis on outcome after extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2007;133:111-6. [Crossref] [PubMed]
- Pilling JE, Stewart DJ, Martin-Ucar AE, et al. The case for routine cervical mediastinoscopy prior to radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2004;25:497-501. [Crossref] [PubMed]
- Tournoy KG, Burgers SA, Annema JT, et al. Transesophageal endoscopic ultrasound with fine needle aspiration in the preoperative staging of malignant pleural mesothelioma. Clin Cancer Res 2008;14:6259-63. [Crossref] [PubMed]
- Bean SM, Eloubeidi MA, Cerfolio R, et al. Endoscopic ultrasound-guided fine needle aspiration is useful for nodal staging in patients with pleural mesothelioma. Diagn Cytopathol 2008;36:32-7. [Crossref] [PubMed]
- Bruno P, Pisani L, Ricci A, et al. Cytology on transbronchial needle aspiration (TBNA): not only for lung cancer. Anticancer Res 2010;30:4769-72. [PubMed]
- Hamamoto J, Notsute D, Tokunaga K, et al. Diagnostic usefulness of endobronchial ultrasound guided transbronchial needle aspiration in a case with malignant pleural mesothelioma. Intern Med 2010;49:423-6. [Crossref] [PubMed]
- Hysi I, Le Pimpec-Barthes F, Alifano M, et al. Lymph node involvement and metastatic lymph node ratio influence the survival of malignant pleural mesothelioma: a French multicenter retrospective study. Oncol Rep 2014;31:415-21. [Crossref] [PubMed]
- Batirel HF, Metintas M, Caglar HB, et al. Adoption of pleurectomy and decortication for malignant mesothelioma leads to similar survival as extrapleural pneumonectomy. J Thorac Cardiovasc Surg 2016;151:478-84. [Crossref] [PubMed]
- de Perrot M, Dong Z, Bradbury P, et al. Impact of tumour thickness on survival after radical radiation and surgery in malignant pleural mesothelioma. Eur Respir J 2017;49: [Crossref] [PubMed]
- Batirel HF, Ak G, Cimsit C, et al. Novel descriptors for clinical staging of mesothelioma.14th International Conference of the International Mesothelioma Interest Group, May 2-5, 2018, Ottawa, Canada.
Cite this article as: Batirel HF. What are the implications of the revised N staging system for surgery? Shanghai Chest 2018;2:71.


