
Patient selection for radical surgery for mesothelioma—prognostic factors in a multimodality approach
Introduction
Research has focused on identification of selection criteria for surgery in a multimodality therapy approach since 1976, when Butchart in Newcastle, UK described the importance of precise patient selection for surgery. He was one of the pioneers in mesothelioma surgery and concluded, after a series of 29 patients undergoing pleuropneumonectomy, that death could have been prevented by better case selection, alteration in surgical technique, and better postoperative management (1). To optimize patient selection, many factors have to be taken into account: the patients’ performance status (PS), the stage of disease, and also availability and access to different treatment approaches. In the following chapter, clinical and pathological staging and prognostic factors will be summarized.
Clinical prognostic factors—fitness for surgery
In general, prognostic factors can be classified into clinical (including hematological/serum) and pathological/molecular features. Butchart divided his cohort in 1976 into “fit” and “unfit for surgery” and categorized them additionally in “above 60 years” or “below 60 years of age” (1). The selection process to decide which patient is eligible for surgery is challenging and requires expertise and interdisciplinary collaboration. The following known risk factors additionally associated with poor prognosis on overall survival (OS) have been identified for patients undergoing surgery for malignant pleural mesothelioma (MPM): age, patient’s comorbidities, weight loss, PS, anemia, high platelet count (PLT), male gender and non-epithelioid subtype (2-7). In one of the first studies by Curran and colleagues, poor PS (Eastern Cooperative Oncology Group PS: ECOG 1 or 2), high white blood cell (WBC) count (>8.3×109/L), probable or possible histologic diagnosis of sarcomatoid subtype, and male gender were in general associated with poor prognosis (4,8,9). Additionally, in a study by van Meerbeeck et al., low level of hemoglobin was a negative prognostic factor for patients undergoing palliative chemotherapy at a hemoglobin difference of ≥1 from the baseline values of 16 g/dL in men and 14 g/dL in women (8).
In general, age is currently a less stringent exclusion criterion for surgery with changing demographics. Age in isolation is not necessarily associated with increased morbidity or mortality as investigated by Williams and colleagues in patients who underwent extended pleurectomy and decortication (EPD) (10). Fitness for surgery needs to be assessed by pulmonary function testing and cardiac assessment (11). There are no clear contraindications for mesothelioma surgery in terms of certain comorbidity profiles. However, the American College of Cardiology and the American Heart Association (ACC/AHA) guidelines established a “Revised Cardiac Risk Index” (RCRI) for a better preoperative risk stratification of patients who need further cardiac evaluations compared to those who are eligible for surgery after basic routine cardiac assessments (12). This RCRI was evaluated in “a patient group undergoing non-cardiac surgery who is at risk for perioperative cardiac morbidity or mortality” based on general recommendations and a review of more than 400 articles. It consists of 6 factors: chronic renal insufficiency, insulin-dependent diabetes mellitus, cerebrovascular disease, heart failure, ischemic heart disease, and high-risk surgical procedure. The common approach for general cardiac stratification starts with basic physical examination and baseline electrocardiography (ECG). ECGs preoperatively are required if the patient would need cardiac assessment even if there is no surgery planned or in case of clinical symptoms or signs of valve/left ventricular dysfunctions, as well as patients with established or documented coronary diseases. Especially, in assessment for extrapleural pneumonectomy (EPP), pulmonary hypertension needs to be excluded, at least by transthoracic echocardiography. If the RCRI is equal or above 2 points or the patient is not able to climb 2 flights of stairs, is on special cardiac medication, or has a newly diagnosed or suspicion of cardiac dysfunction, further assessment according to the AHA/ACC guidelines are necessary. Further tests such as non-invasive stress testing or echocardiography are required for patients at risk.
In conclusion, all of the published “cardiac assessment” scores are intended to give a better risk stratification and should be used as a screening tool and not as a decision-maker. The limitation of these scores is that they were investigated in different patient groups; mostly in patients undergoing lung resection without the differentiation of cancer or non-cancer derived illness. These algorithms should be used with care and should only give the risk profile of the patient. Overall, the decision to undergo surgery or not should still be made individually.
A careful case history and a symptom orientated clinical examination cannot be replaced by any score.
Pre-operative assessments of pulmonary function are equally mandatory for both procedures, EPP or EPD. To our knowledge, there is no mesothelioma specific patient selection concerning the preoperative lung function and only a few reports concerning pulmonary function after EPP or pleurectomy/decortication (P/D) or EPD are reported in literature (13-16). The selection criteria for pneumonectomy for lung cancer can be applied for EPP assessment according to ERS/ESTS guidelines. Even if lung sparing procedure is the initially planned approach, pneumonectomy may become necessary during the operation and therefore feasibility has to be assessed preoperatively. The forced expiratory volume in 1 second (FEV1) for nearly all patients must be greater than 2 L to be able to perform the surgery up to pneumonectomy (16). In addition, quantitative ventilation/perfusion scanning and predicted postoperative (PPO) FEV1 (ideally more than 1.2 L) are necessary to assess for an optimal preoperative diagnostic work up. FEV1 and carbon monoxide lung diffusion capacity (DLCO) should be above 80% for a resection up to pneumonectomy to be feasible (15). Markos and colleagues showed in 1989, that a PPO DLCO of 40% was the best predictor of postoperative respiratory failure, morbidity, and mortality in patients with pulmonary resection up to pneumonectomy (17).
Furthermore, V/Q scan helps to estimate more precisely the PPO FEV1, as in many cases perfusion of the diseased side is dramatically reduced and therefore patients are already before operation “functionally pneumonectomized” (16,18-20). On the other hand, trapped lungs and pleural effusion may influence the lung function assessed in a “false negative way” and can definitely even improve after P/D. The preoperative evaluation of patients with entrapped lung by either pleural effusion or tumor remains challenging, pulmonary lung function tests are not conclusive and have to be interpreted with caution.
Another important factor is the patients’ individual expectancy to postoperative quality of “active” life. For some patients, special hobbies like hiking or playing musical instruments necessitate sufficient lung volume, which is asking for adaptation to the “normal” thresholds of PPO FEV1. This goes in line with the quality of life (QoL) to be expected for every patient after surgery, which needs to be discussed equally on an individual base. Looking at the available literature for QoL after surgery, data are scarce and very heterogeneous (13).
As of today, there are only two observational studies directly comparing QoL after EPP and P/D (14,21). There are many other studies investigating QoL after EPP or P/D without directly comparing the two procedures among each other (13,22,23). Some of these studies demonstrate a tendency for better QoL after lung sparing surgery compared to EPP. These two observational studies concluded that patients undergoing EPP had a greater deterioration in lung function postoperatively and consequently in QoL compared to patients undergoing EPD up to 12 months after surgery. This might be related to the fact that with decortication of entrapped lungs, lung function and therefore QoL can improve.
Pathological and molecular prognostic factors
The first TNM staging system for mesothelioma was proposed by the International Mesothelioma Interest Group (IMIG) in 1994 (24). Since then, this staging system has been revised several times (2,25,26). The IASLC Mesothelioma Staging Project utilized data from 29 centers worldwide, each providing post-operative data, in order to develop the 8th edition of the TNM classification. This revised TNM staging system affects the patient selection in order to increase the number of lower stages suitable for surgery without compromising OS, because in multivariate analyses, there was no significant survival benefit between stage I and II (25). Overall, patients with former advanced diseases according to the old TNM-classification, not feasible undergoing surgery, are downstaged through this new TNM-classification resulting in an increased resectability of MPM. There are no precise selection criteria based on T-factors alone, as clinical staging is too imprecise (27-29). For a better evaluation of resectability in terms of tumor load, tumor volume is an attractive parameter. As described first in 1998 by Pass and colleagues, and later also by us, preoperatively assessed tumor volume is representative of T-status in MPM (30) and can predict overall and progression-free survival, as well as postoperative stage (27). In that same study they showed that large volumes are associated with nodal spread as well as post resection residual tumor burden and may predict outcome (Figure 1) (27,30-32). In another study, Gill et al. showed an association of tumor volume and OS (independently), especially if the tumor volume was greater than 500 cm3. This means, patients with a tumor volume greater than 500 cm3 showed a poorer OS (33).
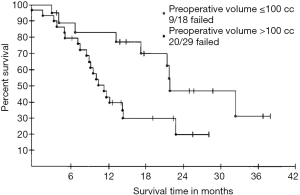
Further evaluation in a multicenter network by Rusch and colleagues, showed a correlation between tumor volume and pT/pN stages plus OS. Within this analysis they defined three groups with tumor volumes of 91.2, 245.3, and 511.3 cm3 and showed that these tumor volumes were associated with a median OS of 37, 18, and 8 months, respectively (32).
In the last proposals for revision of the T Descriptors, it was demonstrated that survival correlated with pleural thickness. Pleural thickness showed an increase at higher T-stages and it was significantly associated with node positivity and overall stage. Based on these findings, data from the seventh edition of T-categories and overall stage, survival showed a median survival of 23.4 months for the lowest tumor thickness (<16.0 mm) compared to a median survival of 13.2 months for the highest tumor thickness (>50.0 mm) (26). Nevertheless, further investigations as to whether tumor thickness should be included in future staging systems are necessary.
Another prognosticator is the status of mediastinal lymph node involvement (N-factor). The current and former editions of N-descriptor of the TNM-classification are based on the lung cancer staging system and are therefore not MPM-specific. The general applicability of lymph node involvement in MPM patients is not clear, as the lymphatic drainage in the two cancers is different. Mesothelioma is primarily a parietal pleura disease, N2 rather than N1 nodes may potentially be the first draining nodal station for this tumor, so hilar or peribronchial node involvement may carry a less favorable prognosis than paratracheal or subcarinal nodes (34). Former N2 is now N1 and N3 is now N2 with the rationale that affected subcarinal, ipsilateral mediastinal lymph nodes as well as lymph nodes from the mammary drainage area do not have a significant effect on OS. Intrapleural and extrapleural lymph node stations are incorporated with the conclusion, that N1 lymph nodes become secondarily involved only when invasion of lung parenchyma occurs (35). Besides IASLC data, poor reliability of N-descriptor on survival was demonstrated in several studies. In a study by Hysi et al. nodal involvement regardless of N1 or N2, significantly reduced the median survival as it was 12.7 months for N+ (N1–N2) staged patients compared to 22.4 months for N0 staged patients. The number of involved or total removed lymph nodes was not related to OS (36). On the contrary, in a study by Edwards et al., the number of positive nodes correlated with survival. The number of involved stations and their anatomic location however did not. Nevertheless, this study also showed that N2 metastases were associated with reduced survival, but as in the study by Hysi et al. there was no difference between N1 and N2 cases (37). In conclusion, this means that nodal involvement seems to be of prognostic importance but the location in the mediastinum has to be interpreted differently from lung cancer. Therefore, the N-descriptor was revised for the eighth TNM-classification to more MPM-specific N-categories in comparison to the seventh edition system, where the median survival for cN0 patients was 19 months versus 17.6 and 16.2 months for cN1 and cN2 patients, respectively and without statistical significance (35).
Distant metastases (M1) can be detected by 18F-fluoro-2-deoxy-D-glucose positron-emission tomography (FDG-PET) scans with an accuracy of 90.3% according to a study by Yildirim et al. (38,39). In the proposal for the eighth edition for TNM-classification in MPM patients, distant metastases were divided into two categories: single metastasis or single site of metastatic disease compared to multiple lesions or sites. After revision of the MPM TNM-classification, the categorization of distant metastases appears to be associated with a different OS (40). Obviously, these patients should not be included into surgery protocols.
Concluding, an improved staging system will also contribute to an improved conformity between different medical centers in respect to a more precise comparison of patient’s selection and outcome (41).
The prognostic impact of many pathological or molecular markers is not fully clarified yet. The main goal is an early detection, which leads to early diagnosis, hence early treatment. The histological subtypes, for example, help to identify patients eligible for surgery or not. The newest ASCO guidelines 2018 by Kindler and colleagues confirm the known better OS (19 months) for patients with epithelioid subtype undergoing surgery compared to patients with biphasic or sarcomatoid subtype with an OS of 12 and 4 months, respectively (42,43). Biphasic or sarcomatoid subtype with negative mediastinal lymph node status, however, should not be excluded from surgery, because this can be the only option for these patients in terms of their higher incidence of chemoresistance (44-46). In conclusion, the decision for or against surgery should not be based on a single factor as histotype, but rather respect a combination of various factors, as there are certain cases of biphasic or sarcomatoid histotypes with long term survival (47,48).
In the following, we will discuss briefly a few molecular prognosticators being a focus of our research area, but not in depth as this is the topic of following chapters.
PD-L1 as a novel target for molecular findings with a high interdisciplinary popularity nowadays, may predict the behavior of MPM, and is possibly associated with a lower median survival according to a newly released study by Nguyen et al. (49). In this study, a positivity of PD-L1 was associated with a median survival of 6 months compared to survival associated with negative PD-L1 expression of 15.5 months.
There are two recently published reviews by Chen et al. and Sun et al., groups from the University of Hawaii Cancer Center and NYU Langone Medical Center (50,51). The authors listed the most promising markers associated with diagnosis and prognosis of MPM in the current research field. Those are, soluble mesothelin-related proteins (SMRPs), osteopontin, Fibulin-3, high-mobility group box 1 (HMGB 1), micro-RNA (miRNA), proteomics, and peripheral blood-based markers [lymphocyte-to-monocyte ratio (LMR), neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR)]. These markers—so far—do not have an impact in patient selection suitable for multimodality therapy approaches. All of them will be briefly discussed below.
SMRP is the most studied and the only Food and Drug Administration (FDA)-approved biomarker, which can be additionally used for monitoring (52). It consists of two major proteins. One part of it is released into the blood and the other part is a plasma membrane-bound protein (53-56). The prognostic value is still controversially discussed and is not yet finally clarified.
Osteopontin is a circulating glycoprotein in the serum and more established as a diagnostic biomarker than having a prognostic impact (57). Pass et al. showed that high osteopontin levels were associated with poorer prognosis after adjusting these markers to the European Organisation for Research and Treatment of Cancer (EORTC) prognostic index (PI). This was proven in a final prognostic model (58).
Fibulin-3, also a glycoprotein and encoded by epidermal growth factor (EGF)-containing fibulin-like extracellular matrix protein-1 (EFEMP-1) gene, is a valuable biomarker for MPM diagnostics, but has not yet been investigated in a prospective manner (59,60). Kirschner et al. (59) demonstrated in two independent cohorts that low levels of fibulin in pleural effusion were associated with better survival (hazard ratio of 9.92).
HMGB 1 is a damage associated molecular pattern (DAMP) protein and released by necrotic cells. Besides its role as a diagnostic marker, it also has prognostic value (61,62), with an inverse association between HMGB 1 serum levels and survival at a cut off value of 9 ng/mL.
MicroRNAs are small non-coding RNA molecules, which regulate gene expression (63,64). So far, 2,588 human miRNAs have been identified. Kirschner et al. (65) established a miR-Score consisting of 6 miRNAs (miR-21-5p, miR-23-3p, miR-30e-5p, miR-221-3p, miR-222-3p) predicting longer survival in positive patients.
Proteomics represents a technique to screen serological markers of the proteome. Proteome is the entire set of proteins expressed by an organism at a certain time. They are only marginally investigated so far and therefore not further discussed in this paper.
A recently published meta-analysis of the prognostic significance of NLR ratio in patients treated surgically and non-surgically also concluded that NLR might have a prognostic role as biomarker in MPM (66).
Lastly, the most promising hematological marker is the peripheral blood-based marker LMR given its proven correlation with survival. Yamagishi and colleagues showed that patients with a LMR serum level greater than 2.74 had an improved OS of 14 months versus 5 months (67) for patients with a lower serum level. For the other blood markers like NLR and PLR, further studies are needed to show their influence as prognostic markers.
Other molecular prognosticators have to be mentioned and will be discussed below, such as chromosome alterations of CDKN2A locus (9p21.3), homozygous p16 deletions (especially for sarcomatous type), and BAP1 mutations. All of them are associated with poor prognosis (68-70).
BRCA1-associated protein-1 (BAP1), a tumor suppressor, is highly discussed lately as a potential prognosticator for MPM, but the prognostic value of somatic BAP1 mutations remains to be evaluated.
Another large study demonstrated that BAP1, NF2, and CDKN2A, were the three most frequently altered genes. CDKN2A mutations occurred in 10 of 22 MPM samples and were the most common event (71) of all gene alterations.
Our group investigated the influence of phosphatase and tensin homologue (PTEN) and its pathways on OS of mesothelioma patients (72,73). The data showed, that high p-S6 and Ki-67 expression in treatment naïve patients was associated with shorter progression-free survival. This, as well as a shorter OS, also applies for high Ki-67 expression after chemotherapy. In addition, they observed that a decrease in PTEN and an increase in p-mTOR expression during induction chemotherapy were associated with shorter OS (73). Furthermore, Meerang et al. discovered that low Merlin expression and high Survivin expression are also associated with a poorer prognosis (74). They showed that Ki-67 and a high nuclear Survivin labeling index in pre- and post-chemotherapy tissues were associated with shorter freedom from recurrence (73,74).
In summary, while all of these markers showed promising results, most have yet to be independently and prospectively validated, and for some of them controversial results from different studies concerning their prognostic impact need to be addressed (51). So far, all these above-mentioned markers and their potential prognostic value have no impact in patient selection, but may be included in selection algorithms alongside with clinical markers in prognostic scores.
Prognostic scores
For clinical use, several groups tried to combine independent prognostic factors to create a score for easier and better risk stratification (69,75-77). The EORTC and the Cancer and Leukaemia Group B (CALGB) developed a prognostic score for a better identification of patients, receiving different chemotherapy regimens, by analyzing the patient’s pretreatment characteristics (4,5). Lactate dehydrogenase (LDH) >500 IU/L, poor PS, chest pain, PLT >400,000/µL, non-epithelial histology, and increasing age older than 75 years jointly predict poor survival and are predictive of a greater risk of dying early. The median survival for PS 2, 1, and 0 was 3.3, 7.6, and 10.9 months respectively, for presence of chest pain 5.4 compared to 8.8 months without chest pain, for PLT above the cut off limit 6.2 compared to 9.4 months, for weight loss 5.1 months compared to 7.9 months for no weight loss, for LDH 3.4 months compared to 7.6 months with low LDH (5). This score was validated in a retrospective study in patients undergoing surgery followed by chemotherapy regimens and eventually radiotherapy or undergoing a palliative procedure followed by chemotherapy, by the Italian group of Ruffini and colleagues and proved to be an independent prognostic factor (75). Edwards and colleagues also validated the effectiveness of the EORTC and CALGB systems. The patient population was heterogeneous, some patients only had surgical biopsy, some underwent surgical resection and in 23 cases, it was not possible to determine the surgical procedure. They had similar survivals for patients stratified into low risk and high-risk groups and correlated with the EORTC series with a median survival of 9.4 vs. 10.8 months (low-risk group) and 3.8 vs. 5.5 months (high-risk group), respectively (3,6,30,78). Shortly after the EORTC and CALGB Score, Pass, Rusch and colleagues identified covariates that were prognostically important for prediction of survival. These variables were stage, age, sex, histology (epithelioid vs. non-epithelioid), and the type of surgical procedure (palliative vs. EPP or P/D) and defined as “CORE” values (79). Additionally to these CORE variables, which were independently statistically significant, they analyzed their impact on OS: adjuvant therapy (yes: OS 18 months vs. no: OS 10 months), smoking history (no: OS 16 months vs. yes: OS 15 months), history of asbestos exposure (no: OS 17 months vs. yes: OS 15 months), history of weight loss, defined as greater than 5% (OS 11 months) vs. less than 5% (OS 17 months) in the previous 6 months, ECOG PS 0 (OS 22 months) and 1 (OS 16 months), chest pain (no: OS 19 months vs. yes: OS 14 months), and dyspnea (no: OS 15 months vs. yes: OS 17 months) as well as laboratory parameters [hemoglobin level (<14.6 OS 16 months vs. >14.6 OS 20 months), PLT (<400 OS 19 months vs. >400 OS 12 months), WBC count (<15.5 OS 16 months vs. >15.5 OS 8 months), and LDH level before the attempted surgical procedures). In total, they defined three prognostic models with these covariates. Models 1 and 2 in common is the inclusion of the CORE variables. Model 1 additionally included adjuvant treatment, WBC count, and platelets. Further in the analysis with stepwise regression all covariates with the least significance were excluded until only significant covariates remained and this led to models 2 and 3. Model 2 consists of the same covariates as model 1, but without a surgical proofed staging. Therefore, instead of calling it the best pathological stage it was changed to the term clinical stage. Furthermore, hemoglobin was added as additional laboratory parameter. Model 3 shows only parameters available before surgery (histology, sex, age, WBC count, hemoglobin, and platelets) and therefore simulates the potential surgical patient. Unfortunately, the models per se were not analyzed as to their prognostic value, only their covariates were individually indicated with their prognostic significance and hazard ratio (79,80).
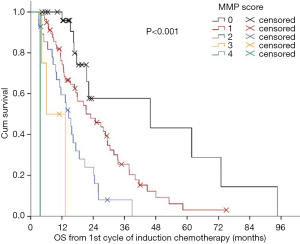
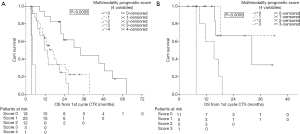
In 2012, we defined a new multimodality prognostic score, only using parameters being available before surgery to support decision making. The items in the score consisted of tumor volume before chemotherapy (>500 mL), non-epithelioid histological diagnosed before chemotherapy, CRP >30 mg/L before chemotherapy, and progressive disease (PD) after chemotherapy. The cutoff within this score was at 2. The median OS for patients with a score of 0, 1, and 2 was 34, 17 and 12 months respectively. Whereas patients with a score of 3 or 4 had a median OS of 4 months (Figure 2). This score was further validated in an independent cohort of patients from a center in Vienna (Figure 3). The comparison of MMPS score with EORTC score using ROC analysis at 2 years showed that the MMPS demonstrated a better predictive power for OS than the EORTC score (81).
Allocation to P/D, EPD or EPP
There is a notable transition in surgery for MPM from EPP to EPD (82). Crucial for this shift was a rethinking after the MARS trial study. EPP was associated with mortality that was not seen with chemo alone, although the study was not designed to test the benefit of EPP (83,84). A follow up study “MARS 2: A Feasibility Study Comparing (Extended) Pleurectomy Decortication Versus no Pleurectomy Decortication in Patients With Malignant Pleural Mesothelioma (MARS2)” (NCT02040272) was satisfactorily completed and the phase III trial is underway in the United Kingdom. Other studies tried to demonstrate survival differences between EPP and extended EPD with a trend towards the less radical lung sparing procedure. The main drawback of EPP is the higher mortality rate compared to EPD (85). The OS also tends to be superior for patients undergoing EPD compared to EPP at least for early stages (82,86-88).
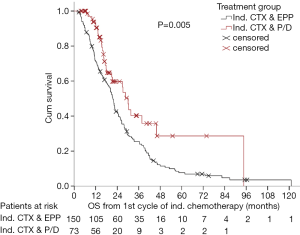
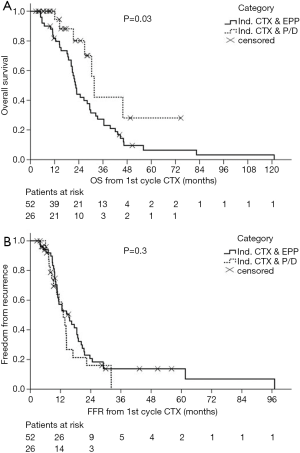
After many years of tradition in performing EPP for MPM patients, our institution shifted towards EPD in more than 90% of the cases with similar survival outcomes (Figures 4,5) [(89) and updated unpublished data] and very low mortality rates after EPD (0%). Generally, mortality rates are certainly lower after EPD as described in a systematic review by Cao et al. of 2.9% compared to 6.8% after EPP. Morbidity rates are as high as after EPP, when prolonged air leaks are taken into account, if not, morbidity rates for EPD are lower with 27.9% compared to 62.0% undergoing EPP (88).
To date, we always aim for a lung sparing procedure. However, there are still situations where EPP might be the better procedure or even the only possible procedure due to the given tumor load. The challenge is to identify these cases upfront surgery, and therefore, in most cases, the decision is taken only during the operation. In case of unambiguous, deep lung tissue infiltration at multiple locations, or central/hilar tumor mass infiltrating hilar vessels, pneumonectomy will be performed. Preoperatively, these situations are hardly detectable by CT scan or PET/CT scan.
Based on restaging imaging by contrast enhanced CT scan or PET/CT after induction chemotherapy and assessment of modified Response Evaluation Criteria in Solid Tumors (mRECIST) (90), patients are classified as PD, stable disease, or partial response and if they are potentially resectable or not. mRECIST is a method most widely used for measurements in solid tumors. It is based on “unidimensional measurements of tumor thickness perpendicular to the chest wall or mediastinum” (90,91). Because of the difficulties in reproducibility, we are additionally using the delta of pre- and post-chemotherapy volume assessed by a dedicated software (27) (Figure 6). PD alone is not an exclusion criteria per se for surgery as long as macroscopic complete resection is still feasible. In some cases, further radiological assessments with magnetic resonance imaging (MRI) and functional imaging with FDG-PET need to be conducted to rule out chest wall infiltration, transdiaphragmatic infiltration, or nodal involvement and occult metastasis (39,92). Time between last cycle of induction chemotherapy and surgery should not exceed 6 weeks post induction chemotherapy (93). Obviously, all patients should be discussed in an interdisciplinary tumor board consisting of pulmonologists, radiologists, radio-oncologists, oncologists, pathologists, and thoracic surgeons experienced in mesothelioma resections. Tumor stages I–III and all histologies are included if deemed technically resectable, with most of the patients treated within clinical trial protocols. Decisions leading to surgery are made individually and even localized chest wall infiltration is accepted if chest wall resection seems feasible and reasonable at only one level. Furthermore, our MMPs score is used as exclusion criterion (MMPS ≤2). The procedure of choice is EPD if technically and oncologically feasible (82,86,87,94). Only in cases of multiple spots with direct lung parenchyma invasion, infiltration of central structures including great vessels (Figure 7) or bronchus and if the patient’s condition and preoperative lung function tests allow it, we conduct an EPP. On the contrary, if macroscopic complete resection cannot be achieved (R2), a partial pleurectomy is usually performed, without resection of the pericardium or diaphragm.
Conclusions
Currently, there are no generally accepted criteria for patient selection for surgery within a multimodality treatment protocol, but patient selection remains the key role for surgical success and low morbidity and mortality. Amongst clinical, pathological, and molecular prognostic factors studied in the past, the combination of several factors seems to be the most appropriate tool for decision-making including also institutional experience and availability of therapy as well as factors such as the patients’ treatment choices.
At our institution, selection of candidates for surgery after induction chemotherapy is based on the following factors: Patients of all histological subtypes are eligible for surgery within clinical trial protocols, tumor stage T1–T3, N0–N2 confirmed by mediastinal staging, deemed technically resectable by a thoracic surgeon experienced in mesothelioma surgery, MMPS ≤2, fit enough for surgery in terms of their PS (0–1) and comorbidities and functional reserve (including optional EPP).
Obviously, discussion in a multidisciplinary tumor board will help to include many aspects in this decision-making process and prospective evaluation of these treatment allocation protocols will help to select the ideal fitting therapy for our patients in the future. The final decision for EPP or EPD is taken in most of the cases based on the intraoperative findings based on the degree of lung tissue infiltration.
Acknowledgments
This work was supported by a Swiss National Science Foundation Professorship to Isabelle Opitz. We want to thank Mayura Meerang and Michaela Kirschner for critical review of the molecular biomarker paragraph, Martina Friess for critical review of the clinical paragraph and edition of figures, and Chloé Spichiger for editing of the manuscript.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (David Waller and Annabel Sharkey) for the series “Mesothelioma Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.09.01). The series “Mesothelioma Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Butchart EG, Ashcroft T, Barnsley WC, et al. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976;31:15-24. [Crossref] [PubMed]
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Edwards JG, Martin-Ucar AE, Stewart DJ, et al. Right extrapleural pneumonectomy for malignant mesothelioma via median sternotomy or thoracotomy? Short- and long-term results. Eur J Cardiothorac Surg 2007;31:759-64. [Crossref] [PubMed]
- Curran D, Sahmoud T, Therasse P, et al. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol 1998;16:145-52. [Crossref] [PubMed]
- Herndon JE, Green MR, Chahinian AP, et al. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest 1998;113:723-31. [Crossref] [PubMed]
- Edwards JG, Abrams KR, Leverment JN, et al. Prognostic factors for malignant mesothelioma in 142 patients: validation of CALGB and EORTC prognostic scoring systems. Thorax 2000;55:731-5. [Crossref] [PubMed]
- Gonlugur U, Gonlugur TE. Prognostic factors for 100 patients with malignant pleural mesothelioma. Arch Environ Occup Health 2010;65:65-9. [Crossref] [PubMed]
- van Meerbeeck JP, Gaafar R, Manegold C, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 2005;23:6881-9. [Crossref] [PubMed]
- Ceresoli GL, Grosso F, Zucali PA, et al. Prognostic factors in elderly patients with malignant pleural mesothelioma: results of a multicenter survey. Br J Cancer 2014;111:220-6. [Crossref] [PubMed]
- Williams T, Duraid H, Watson S, et al. Extended Pleurectomy and Decortication for Malignant Pleural Mesothelioma Is an Effective and Safe Cytoreductive Surgery in the Elderly. Ann Thorac Surg 2015;100:1868-74. [Crossref] [PubMed]
- Sharkey AJ, Bilancia R, Tenconi S, et al. Extended pleurectomy decortication for malignant pleural mesothelioma in the elderly: the need for an inclusive yet selective approach. Interact Cardiovasc Thorac Surg 2017;25:696-702. [Crossref] [PubMed]
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. J Am Coll Cardiol 2007;50:e159-241. Erratum in: J Am Coll Cardiol 2007;50:e242 J Am Coll Cardiol 2008;52:793-4. Chaikof, Elliott [corrected to Chaikof, Elliott L]. [Crossref] [PubMed]
- Schwartz RM, Watson A, Wolf A, et al. The impact of surgical approach on quality of life for pleural malignant mesothelioma. Ann Transl Med 2017;5:230. [Crossref] [PubMed]
- Ploenes T, Osei-Agyemang T, Krohn A, et al. Changes in lung function after surgery for mesothelioma. Asian Cardiovasc Thorac Ann 2013;21:48-55. [Crossref] [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-e190S. Erratum in: Chest 2014;145:437.
- Markos J, Mullan BP, Hillman DR, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis 1989;139:902-10. [Crossref] [PubMed]
- Colice GL, Shafazand S, Griffin JP, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:161S-77S.
- Brunelli A, Refai M, Xiume F, et al. Performance at symptom-limited stair-climbing test is associated with increased cardiopulmonary complications, mortality, and costs after major lung resection. Ann Thorac Surg 2008;86:240-7; discussion 247-8. [Crossref] [PubMed]
- British Thoracic Society. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 2001;56:89-108. [Crossref] [PubMed]
- Rena O, Casadio C. Extrapleural pneumonectomy for early stage malignant pleural mesothelioma: a harmful procedure. Lung Cancer 2012;77:151-5. [Crossref] [PubMed]
- Ambrogi V, Mineo D, Gatti A, et al. Symptomatic and quality of life changes after extrapleural pneumonectomy for malignant pleural mesothelioma. J Surg Oncol 2009;100:199-204. [Crossref] [PubMed]
- Tanaka T, Morishita S, Hashimoto M, et al. Physical function and health-related quality of life in patients undergoing surgical treatment for malignant pleural mesothelioma. Support Care Cancer 2017;25:2569-75. [Crossref] [PubMed]
- Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma from the International Mesothelioma Interest Group. Lung Cancer 1996;14:1-12. [Crossref] [PubMed]
- Pass H, Giroux D, Kennedy C, et al. The IASLC Mesothelioma Staging Project: Improving Staging of a Rare Disease Through International Participation. J Thorac Oncol 2016;11:2082-8. [Crossref] [PubMed]
- Nowak AK, Chansky K, Rice DC, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2089-99.
- Frauenfelder T, Tutic M, Weder W, et al. Volumetry: an alternative to assess therapy response for malignant pleural mesothelioma? Eur Respir J 2011;38:162-8. [Crossref] [PubMed]
- Armato SG 3rd, Blyth KG, Keating JJ, et al. Imaging in pleural mesothelioma: A review of the 13th International Conference of the International Mesothelioma Interest Group. Lung Cancer 2016;101:48-58. [Crossref] [PubMed]
- Lee HY, Hyun SH, Lee KS, et al. Volume-based parameter of 18)F-FDG PET/CT in malignant pleural mesothelioma: prediction of therapeutic response and prognostic implications. Ann Surg Oncol 2010;17:2787-94. [Crossref] [PubMed]
- Pass HI, Temeck BK, Kranda K, et al. Preoperative tumor volume is associated with outcome in malignant pleural mesothelioma. J Thorac Cardiovasc Surg 1998;115:310-7; discussion 317-8. [Crossref] [PubMed]
- Sensakovic WF, Armato SG 3rd, Straus C, et al. Computerized segmentation and measurement of malignant pleural mesothelioma. Med Phys 2011;38:238-44. [Crossref] [PubMed]
- Rusch VW, Gill R, Mitchell A, et al. A Multicenter Study of Volumetric Computed Tomography for Staging Malignant Pleural Mesothelioma. Ann Thorac Surg 2016;102:1059-66. [Crossref] [PubMed]
- Gill RR, Richards WG, Yeap BY, et al. Epithelial malignant pleural mesothelioma after extrapleural pneumonectomy: stratification of survival with CT-derived tumor volume. AJR Am J Roentgenol 2012;198:359-63. [Crossref] [PubMed]
- Sugarbaker DJ, Strauss GM, Lynch TJ, et al. Node status has prognostic significance in the multimodality therapy of diffuse, malignant mesothelioma. J Clin Oncol 1993;11:1172-8. [Crossref] [PubMed]
- Rice D, Chansky K, Nowak A, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the N Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2100-11.
- Hysi I, Le Pimpec-Barthes F, Alifano M, et al. Lymph node involvement and metastatic lymph node ratio influence the survival of malignant pleural mesothelioma: a French multicenter retrospective study. Oncol Rep 2014;31:415-21. [Crossref] [PubMed]
- Edwards JG, Stewart DJ, Martin-Ucar A, et al. The pattern of lymph node involvement influences outcome after extrapleural pneumonectomy for malignant mesothelioma. J Thorac Cardiovasc Surg 2006;131:981-7. [Crossref] [PubMed]
- Yildirim H, Metintas M, Entok E, et al. Clinical value of fluorodeoxyglucose-positron emission tomography/computed tomography in differentiation of malignant mesothelioma from asbestos-related benign pleural disease: an observational pilot study. J Thorac Oncol 2009;4:1480-4. [Crossref] [PubMed]
- Martini K, Meier A, Opitz I, et al. Diagnostic accuracy of sequential co-registered PET+MR in comparison to PET/CT in local thoracic staging of malignant pleural mesothelioma. Lung Cancer 2016;94:40-5. [Crossref] [PubMed]
- Rusch VW, Chansky K, Kindler HL, et al. The IASLC Mesothelioma Staging Project: Proposals for the M Descriptors and for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Mesothelioma. J Thorac Oncol 2016;11:2112-9. [Crossref] [PubMed]
- De Laet C, Domen A, Cheung KJ, et al. Malignant Pleural Mesothelioma: Rationale for a New TNM Classification. Acta Chir Belg 2014;114:245-9. [PubMed]
- Kindler HL, Ismaila N, Armato SG 3rd, et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1343-73. [Crossref] [PubMed]
- Meyerhoff RR, Yang CF, Speicher PJ, et al. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J Surg Res 2015;196:23-32. [Crossref] [PubMed]
- Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54-63; discussion 63-5. [Crossref] [PubMed]
- Musk AW, Olsen N, Alfonso H, et al. Predicting survival in malignant mesothelioma. Eur Respir J 2011;38:1420-4. [Crossref] [PubMed]
- Saddoughi SA, Abdelsattar ZM, Blackmon SH. National Trends in the Epidemiology of Malignant Pleural Mesothelioma: A National Cancer Data Base Study. Ann Thorac Surg 2018;105:432-7. [Crossref] [PubMed]
- Nakas A, Trousse DS, Martin-Ucar AE, et al. Open lung-sparing surgery for malignant pleural mesothelioma: the benefits of a radical approach within multimodality therapy. Eur J Cardiothorac Surg 2008;34:886-91. [Crossref] [PubMed]
- Neragi-Miandoab S, Richards WG, Sugarbaker DJ. Morbidity, mortality, mean survival, and the impact of histology on survival after pleurectomy in 64 patients with malignant pleural mesothelioma. Int J Surg 2008;6:293-7. [Crossref] [PubMed]
- Nguyen BH, Montgomery R, Fadia M, et al. PD-L1 expression associated with worse survival outcome in malignant pleural mesothelioma. Asia Pac J Clin Oncol 2018;14:69-73. [Crossref] [PubMed]
- Chen Z, Gaudino G, Pass HI, et al. Diagnostic and prognostic biomarkers for malignant mesothelioma: an update. Transl Lung Cancer Res 2017;6:259-69. [Crossref] [PubMed]
- Sun HH, Vaynblat A, Pass HI. Diagnosis and prognosis-review of biomarkers for mesothelioma. Ann Transl Med 2017;5:244. [Crossref] [PubMed]
- Canessa PA, Franceschini MC, Ferro P, et al. Evaluation of soluble mesothelin-related peptide as a diagnostic marker of malignant pleural mesothelioma effusions: its contribution to cytology. Cancer Invest 2013;31:43-50. [Crossref] [PubMed]
- Scherpereel A, Grigoriu B, Conti M, et al. Soluble mesothelin-related peptides in the diagnosis of malignant pleural mesothelioma. Am J Respir Crit Care Med 2006;173:1155-60. [Crossref] [PubMed]
- Cristaudo A, Foddis R, Vivaldi A, et al. Clinical significance of serum mesothelin in patients with mesothelioma and lung cancer. Clin Cancer Res 2007;13:5076-81. [Crossref] [PubMed]
- Schneider J, Hoffmann H, Dienemann H, et al. Diagnostic and Prognostic Value of Soluble Mesothelin-Related Proteins in Patients with Malignant Pleural Mesothelioma in Comparison with Benign Asbestosis and Lung Cancer. J Thorac Oncol 2008;3:1317-24. [Crossref] [PubMed]
- Linch M, Gennatas S, Kazikin S, et al. A serum mesothelin level is a prognostic indicator for patients with malignant mesothelioma in routine clinical practice. BMC Cancer 2014;14:674. [Crossref] [PubMed]
- Cappia S, Righi L, Mirabelli D, et al. Prognostic role of osteopontin expression in malignant pleural mesothelioma. Am J Clin Pathol 2008;130:58-64. [Crossref] [PubMed]
- Pass HI, Goparaju C, Espin-Garcia O, et al. Plasma Biomarker Enrichment of Clinical Prognostic Indices in Malignant Pleural Mesothelioma. J Thorac Oncol 2016;11:900-9. [Crossref] [PubMed]
- Kirschner MB, Pulford E, Hoda MA, et al. Fibulin-3 levels in malignant pleural mesothelioma are associated with prognosis but not diagnosis. Br J Cancer 2015;113:963-9. [Crossref] [PubMed]
- Creaney J, Dick IM, Meniawy TM, et al. Comparison of fibulin-3 and mesothelin as markers in malignant mesothelioma. Thorax 2014;69:895-902. [Crossref] [PubMed]
- Wu T, Zhang W, Yang G, et al. HMGB1 overexpression as a prognostic factor for survival in cancer: a meta-analysis and systematic review. Oncotarget 2016;7:50417-27. [PubMed]
- Tabata C, Shibata E, Tabata R, et al. Serum HMGB1 as a prognostic marker for malignant pleural mesothelioma. BMC Cancer 2013;13:205. [Crossref] [PubMed]
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116:281-97. [Crossref] [PubMed]
- Ambros V. The functions of animal microRNAs. Nature 2004;431:350-5. [Crossref] [PubMed]
- Kirschner MB, Cheng YY, Armstrong NJ, et al. MiR-score: a novel 6-microRNA signature that predicts survival outcomes in patients with malignant pleural mesothelioma. Mol Oncol 2015;9:715-26. [Crossref] [PubMed]
- Chen N, Liu S, Huang L, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in patients with malignant pleural mesothelioma: a meta-analysis. Oncotarget 2017;8:57460-9. [PubMed]
- Yamagishi T, Fujimoto N, Nishi H, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with malignant pleural mesothelioma. Lung Cancer 2015;90:111-7. [Crossref] [PubMed]
- Jean D, Daubriac J, Le Pimpec-Barthes F, et al. Molecular changes in mesothelioma with an impact on prognosis and treatment. Arch Pathol Lab Med 2012;136:277-93. [Crossref] [PubMed]
- Baumann F, Flores E, Napolitano A, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis 2015;36:76-81. [Crossref] [PubMed]
- McGregor SM, Dunning R, Hyjek E, et al. BAP1 facilitates diagnostic objectivity, classification, and prognostication in malignant pleural mesothelioma. Hum Pathol 2015;46:1670-8. [Crossref] [PubMed]
- Guo G, Chmielecki J, Goparaju C, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res 2015;75:264-9. [Crossref] [PubMed]
- Opitz I, Soltermann A, Abaecherli M, et al. PTEN expression is a strong predictor of survival in mesothelioma patients. Eur J Cardiothorac Surg 2008;33:502-6. [Crossref] [PubMed]
- Bitanihirwe BK, Meerang M, Friess M, et al. PI3K/mTOR signaling in mesothelioma patients treated with induction chemotherapy followed by extrapleural pneumonectomy. J Thorac Oncol 2014;9:239-47. [Crossref] [PubMed]
- Meerang M, Berard K, Friess M, et al. Low Merlin expression and high Survivin labeling index are indicators for poor prognosis in patients with malignant pleural mesothelioma. Mol Oncol 2016;10:1255-65. [Crossref] [PubMed]
- Sandri A, Guerrera F, Roffinella M, et al. Validation of EORTC and CALGB prognostic models in surgical patients submitted to diagnostic, palliative or curative surgery for malignant pleural mesothelioma. J Thorac Dis 2016;8:2121-7. [Crossref] [PubMed]
- Steele JP. Prognostic factors in mesothelioma. Semin Oncol 2002;29:36-40. [Crossref] [PubMed]
- Richards WG, Godleski JJ, Yeap BY, et al. Proposed adjustments to pathologic staging of epithelial malignant pleural mesothelioma based on analysis of 354 cases. Cancer 2010;116:1510-7. [Crossref] [PubMed]
- Rice DC, Stevens CW, Correa AM, et al. Outcomes After Extrapleural Pneumonectomy and Intensity-Modulated Radiation Therapy for Malignant Pleural Mesothelioma. Ann Thorac Surg 2007;84:1685-92; discussion 1692-3.
- Pass HI, Giroux D, Kennedy C, et al. Supplementary prognostic variables for pleural mesothelioma: a report from the IASLC staging committee. J Thorac Oncol 2014;9:856-64. [Crossref] [PubMed]
- Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol 2012;7:1631-9. [Crossref] [PubMed]
- Opitz I, Friess M, Kestenholz P, et al. A New Prognostic Score Supporting Treatment Allocation for Multimodality Therapy for Malignant Pleural Mesothelioma: A Review of 12 Years' Experience. J Thorac Oncol 2015;10:1634-41. [Crossref] [PubMed]
- Batirel HF, Metintas M, Caglar HB, et al. Adoption of pleurectomy and decortication for malignant mesothelioma leads to similar survival as extrapleural pneumonectomy. J Thorac Cardiovasc Surg 2016;151:478-84. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Weder W, Stahel RA, Baas P, et al. The MARS feasibility trial: conclusions not supported by data. Lancet Oncol 2011;12:1093-4; author reply 1094-5. [Crossref] [PubMed]
- Burt BM, Cameron RB, Mollberg NM, et al. Malignant pleural mesothelioma and the Society of Thoracic Surgeons Database: an analysis of surgical morbidity and mortality. J Thorac Cardiovasc Surg 2014;148:30-5. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 626.e1-3.
- Wolf AS, Flores RM. Current Treatment of Mesothelioma: Extrapleural Pneumonectomy Versus Pleurectomy/Decortication. Thorac Surg Clin 2016;26:359-75. [Crossref] [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [Crossref] [PubMed]
- Kostron A, Friess M, Inci I, et al. Propensity matched comparison of extrapleural pneumonectomy and pleurectomy/decortication for mesothelioma patients. Interact Cardiovasc Thorac Surg 2017;24:740-6. [Crossref] [PubMed]
- Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 2004;15:257-60. [Crossref] [PubMed]
- Bonomi M, De Filippis C, Lopci E, et al. Clinical staging of malignant pleural mesothelioma: current perspectives. Lung Cancer (Auckl) 2017;8:127-39. [Crossref] [PubMed]
- Wang ZJ, Reddy GP, Gotway MB, et al. Malignant pleural mesothelioma: evaluation with CT, MR imaging, and PET. Radiographics 2004;24:105-19. [Crossref] [PubMed]
- Weder W, Kestenholz P, Taverna C, et al. Neoadjuvant Chemotherapy Followed by Extrapleural Pneumonectomy in Malignant Pleural Mesothelioma. J Clin Oncol 2004;22:3451-7. [Crossref] [PubMed]
- Flores RM. Pleurectomy decortication for mesothelioma: The procedure of choice when possible. J Thorac Cardiovasc Surg 2016;151:310-2. [Crossref] [PubMed]
Cite this article as: Lauk O, Opitz I. Patient selection for radical surgery for mesothelioma—prognostic factors in a multimodality approach. Shanghai Chest 2018;2:73.


