Surgical approaches for extrapleural pneumonectomy for mesothelioma: the lower door thoracotomy
Introduction
Malignant pleural mesothelioma (MPM) is a highly aggressive malignancy of the pleura associated with a median survival of 4 to 19 months only (1-3). The incidence of MPM worldwide is underestimated and increasing, as suggested by epidemiologic studies (4,5).
The optimal treatment for patients with MPM is still objected of controversy in the last decades (6): it includes surgery, systemic chemotherapy and radiation. The lack of evidence for answering this specific topic is mainly because MPM is a relatively rare disease with a very heterogenic clinical presentation and a shockingly unpredictable biological behaviour.
Treatment of MPM with radical intent is not focused on surgical resection alone; it includes multimodal approaches involving chemotherapy with neoadjuvant intent followed by extrapleural pneumonectomy (EPP) or pleurectomy/decortication (P/D) and adjuvant radiotherapy.
Multimodal therapy, when patients are appropriately selected, produces better results compared with other strategies alone. However, the precise role the role of surgery in the treatment of MPM is controversial (7) and although complete macroscopic resection is thought to improve long-term survival (3), a surgical standard of care for patients with MPM has not been yet established.
EPP consists in en bloc resection of parietal pleura, lung, pericardium and diaphragm, followed by pericardial and diaphragmatic reconstruction. P/D includes resection of the parietal and visceral pleura, but without removal of the entire lung. Pericardial or diaphragmatic resection is usually considered part of the P/D procedure.
When planning surgery for patients with MPM, the choice of EPP instead of P/D is established evaluating several factors: tumour burden, distribution of disease, institutional factors, surgeon preference and experience in particular.
EEP undoubtedly allows to achieve the optimal ideal of surgical radicality, but mortality and morbidity rates are necessarily higher compared to P/D, although it is likely that operative volume and surgeon experience should influence morbidity and mortality outcomes after EPP. Proponents of each procedure assert the benefit regarding oncologic and technical advantages of a specific intervention (EEP or P/D), but to date, no clear advantage in terms of long-standing survival of one over the other has been shown (8).
EPP
As maximum cytoreduction provided by complete resection was thought as the critical factor to long-term survival for MPM patients, EPP was considered for many years to be the only surgical procedure capable of obtaining a complete macroscopic resection, and it was applied to all operable patients.
EPP is a sophisticated, high demanding intervention that necessarily requires only surgical centres with consolidated experience and volume. It consists in the radical en bloc resection of the entire lung, pleura, pericardium and diaphragm, and subsequent reconstruction with a prosthetic patch.
The operative technique includes two main steps: a demolition and a reconstructive step.
Demolition step
After incision and exposure of the surgical field, extrapleural dissection is conducted by separating tumour from the chest wall using blunt and sharp dissection alternately. Dissection initiates posterolaterally towards the apex and continues inferiorly, laterally and posteriorly in order to reach distal insertions of diaphragm into the chest wall. Tumour invasion of the chest wall is suspected when skeletal muscle is found on the parietal pleura, intra-operative histology is mandatory for confirmation.
The pericardium is incised on the anterior surface and digital exploration of the pericardial space is carried out to exclude direct myocardial invasion. Lateral diaphragmatic margin is incised first, subsequently circumferential resection is conducted anteriorly and posteriorly. Particular care is given to recognition and preparation of inferior vena cava and oesophageal hiatus. Peritoneum is bluntly divided from the abdominal surface of the diaphragm.
Pericardiotomy is extended superiorly, and the main hilar structures (artery, pulmonary veins and bronchus) are divided. The lung is removed “en bloc” with pericardium and diaphragm. Standard lymph node dissection is performed. A pedunculated flap can be prepared to buttress the bronchial stump.
Reconstructive step
Prosthetic patches, generally PTFE or absorbable materials, are used to reconstruct diaphragmatic and pericardial defects to prevent, respectively, herniation of the abdominal viscera or heart into the thoracic cavity.
Diaphragmatic prosthesis is fixed to the chest wall with circumferential sutures. Pericardial fixation is performed in the cut edge of pericardium inferiorly. Medially, prosthesis of diaphragm and pericardium are sutured each other including the cut edge of the native pericardium.
Surgical approaches to EPP
Median sternotomy
When bilateral lung resection has planned the benefit of a medial sternotomy, in comparison with bilateral thoracotomy, is well established (9,10). It includes reduced pain on postoperative course and, consequently, lesser requirements of analgesia, decreased occurrence of complications, faster recovery of postoperative respiratory performance and a hospital stay significantly reduced (11). Sternotomy is often utilized as an effective access in Lung volume reduction surgery.
Sternotomic approach for resections of right lung or the left upper lobe is described in the literature, but a routinely use of median sternotomy for performing right EPP is only occasionally reported. Martin-Ucar (12) et al. report his experience with 33 EPP performed by median sternotomy: with this surgical access they obtained shorter operating times and less analgesic requirement after surgery. They conclude that median sternotomy is a viable alternative to the posterolateral thoracotomy since oncologic result are unchanged regarding the radicality of resection and adequacy of nodal staging. They also noted a survival benefit over thoracotomy.
In opposition, other authors (13) reply stating that sternotomy undoubtedly offers the opportunity to effectively manage the right pulmonary veins and artery an excellent surgical field of the lung apex. However, it is challenging to access the costophrenic angle and paravertebral sulcus when lobectomies or pneumonectomies are performed. Moreover, complete lymphadenectomy of mediastinal stations through this approach could become a true surgical challenge.
Single- or two-level posterolateral thoracotomy
In general thoracic surgery, posterolateral thoracotomy has been the most frequently used incision for several decades. It provides an outstanding access to the lung, hilum, mediastinum, thoracic trachea and oesophagus and consequently it offers the chance to safely control pulmonary blood vessels during complex resections.
Skin incision starts following the fifth or sixth intercostal space at the level of the anterior axillary line and continues curving. Afterwards it curves around the tip of the scapula and continues posteriorly and medially.
The latissimus dorsi is divided, the serratus anterior muscle is usually spared. Most pulmonary resections are performed accessing the pleural space by placing a chest spreader retractor through the fifth intercostal space.
In this fashion posterolateral thoracotomy offers excellent exposure to the pleural space of almost all the areas of the hemithorax than any other standard thoracic single incision.
However, when complete removal of the diaphragm is required, it is difficult to accomplish a radical diaphragmatic resection via standard posterolateral thoracotomy in the fifth intercostals space because of the insufficient operative field for the costo- and cardio-phrenic angles.
For this reason, to extend the surgical field towards costophrenic edges, an additional thoracotomy is usually performed in the ninth or tenth intercostal space.
The lower door (LD) thoracotomy
LD thoracotomy summarises the need to provide a continuous exposure of the operative field during the diaphragm resection. In fact, a second thoracotomy offers an inexorably narrow surgical view (14) since the edge of the diaphragm is necessarily located in the dead angle of the pleural cavity.
LD thoracotomy provides an improved operative field than any other traditional procedure, especially for the costo- and cardio-phrenic angles when the diaphragm is resected, and subsequent prosthetic reconstruction is required.
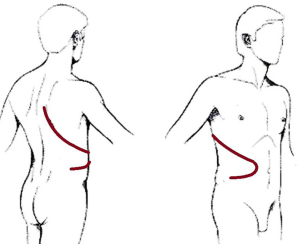
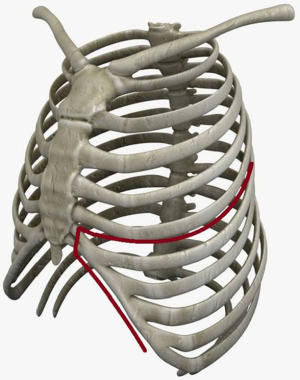
Horio and Nomori first described the technique in 1995 (15). It starts with a standard posterolateral thoracotomy performed at the fifth rib; afterwards skin incision is extended along the anterior costal arch followed by the cutting of the sixth through ninth costal cartilages and the division of the external and internal oblique abdominal muscles below the anterior costal arch. In this fashion, the anterior lower chest wall can be pulled out and posteriorly with a retractor (Figures 1,2).
The anterior section of the lower thorax is consequently opened like a door: this approach provides broad and continuous visibility of the pleural cavity and excellent exposure of both the costo- and cardio-phrenic angles and diaphragmatic edges (14). As a result, the diaphragm can be easily divided with blunt dissection, and underlying peritoneum can be straightforwardly preserved.
All these surgical steps are otherwise very challenging using a single or double thoracotomy as optimal exposure of the surgical field is not assured.
LD subcostal incision can be performed when a surgical indication to EPP is definitively confirmed intraoperatively. This represents a remarkable advantage of this procedure as it can be carried out after a standard posterolateral thoracotomy when LD is required as it does not require individual pre-operative planning.
With this approach en bloc removal of the lung, parietal pleura, pericardium and diaphragm, can be performed radically, accurately and safely.
From November 2005 to February 2017, 15 consecutive patients with malignant pleural epithelial mesothelioma underwent EPP with LD thoracotomy in our Institution.
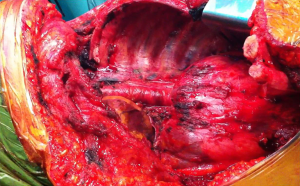
In all the cases we experienced striking advantages regarding extended visual of the surgical field, and consequently, both demolition and the reconstructive step of the diaphragm were carried out more accurately and safely (Figures 3,4).
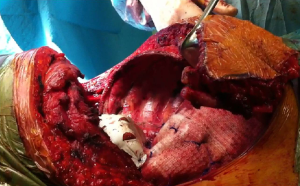
No 30-day mortality occurred, morbidity was substantially in line with the results of literature, we did not report any added mobility specifically related to the LD procedure.
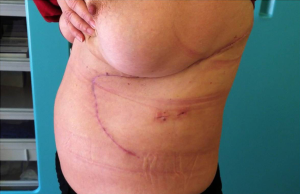
Despite the extent of the surgical access, the cosmetic result was acceptable (Figure 5).
Conclusions
EPP still represents a reliable option in the treatment of MPM. Diaphragmatic resection and reconstruction are doubtless the most challenging passage of EPP because of the limited visibility of the surgical field provided by a standard thoracotomy.
LD thoracotomy, although it is a relatively low demanding procedure, offers outstanding advantages in terms of safeness and efficacy during EPP execution.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Shanghai Chest for the series “Surgical Approaches to VATS Lobectomy: Meet the Experts”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.11.03). The series “Surgical Approaches to VATS Lobectomy” was commissioned by the editorial office without any funding or sponsorship. LS served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Shanghai Chest from Jul 2017 to Jun 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Merritt N, Blewett CJ, Miller JD, et al. Survival after conservative (palliative) management of pleural malignant mesothelioma. J Surg Oncol 2001;78:171-4. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Rusch V, Baldini EH, Bueno R, et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: meeting summary of the International Mesothelioma Interest Group Congress, September 11-14, 2012, Boston, Mass. J Thorac Cardiovasc Surg 2013;145:909-10. [Crossref] [PubMed]
- Park EK, Takahashi K, Hoshuyama T, et al. Global magnitude of reported and unreported mesothelioma. Environ Health Perspect 2011;119:514-8. [Crossref] [PubMed]
- Delgermaa V, Takahashi K, Park EK, et al. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ 2011;89:716-24, 724A-C.
- Opitz I, Weder W. A nuanced view of extrapleural pneumonectomy for malignant pleural mesothelioma. Ann Transl Med 2017;5:237. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 626.e1-3.
- Burt BM, Cameron RB, Mollberg NM, et al. Malignant pleural mesothelioma and the Society of Thoracic Surgeons Database: an analysis of surgical morbidity and mortality. J Thorac Cardiovasc Surg 2014;148:30-5. [Crossref] [PubMed]
- Cooper JD, Nelems JM, Pearson FG. Extended indications for median sternotomy in patients requiring pulmonary resection. Ann Thorac Surg 1978;26:413-20. [Crossref] [PubMed]
- Urschel HC Jr, Razzuk MA. Median sternotomy as a standard approach for pulmonary resection. Ann Thorac Surg 1986;41:130-4. [Crossref] [PubMed]
- Asaph JW, Handy JR Jr, Grunkemeier GL, et al. Median sternotomy versus thoracotomy to resect primary lung cancer: analysis of 815 cases. Ann Thorac Surg 2000;70:373-9. [Crossref] [PubMed]
- Martin-Ucar AE, Stewart DJ, West KJ, et al. A median sternotomy approach to right extrapleural pneumonectomy for mesothelioma. Ann Thorac Surg 2005;80:1143-5. [Crossref] [PubMed]
- Hunt I, Lang-Lazdunski L. Is median sternotomy an appropriate approach to right extrapleural pneumonectomy for mesothelioma?. Ann Thorac Surg 2006;82:767-author reply 767. [Crossref] [PubMed]
- Kameyama K, Huang CL, Hayashi E, et al. Extended posterolateral-subcostal thoracotomy for extrapleural pneumonectomy: a surgical approach for radical operation of pleural mesothelioma. Interact Cardiovasc Thorac Surg. 2004;3:201-3. [Crossref] [PubMed]
- Horio H, Nomori H. Thoracotomy technique for extrapleural pneumonectomy in treatment of diffuse pleural mesothelioma. J Jap Assoc Chest Surg 1995;9:538-41. [Crossref]
Cite this article as: Kawamukai K, Bertolaccini L, Lacava N, Forti Parri SN, Bonfanti B, Solli P. Surgical approaches for extrapleural pneumonectomy for mesothelioma: the lower door thoracotomy. Shanghai Chest 2018;2:86.