
Subxiphoid uniportal video assisted thoracoscopic surgery lobectomy, evolution of the technique and progress of learning curve
Introduction
Video assisted thoracoscopic surgery (VATS) lobectomy has been considered as safe, feasible and effective tool in both benign and early malignant lung diseases (1-4). Evolution from multiport to uniportal VATS lobectomy has been progressing in a way aiming to lessen surgical trauma with reported benefits in favor of uniportal approach (5,6). Liu and his colleagues reported the first uniportal subxiphoid VATS (SVATS) lobectomy in 2014 (7). That has been followed by multiple case series and case reports showing feasibility and safety of the SVATS lobectomy in selected cases with results similar to those of intercostal uniportal VATS approach in terms of duration of chest drain, length of postoperative stay, operative time, conversion rate and complications (8-10). The aim of this study is to further evaluate the uniportal SVATS lobectomy in terms of operative technique and perioperative results with deeper insight into changes associated with the progress in the learning curve.
Methods
Patients
This comparative cross-sectional study was conducted on 438 patients who underwent uniportal SVATS lobectomy from September 2014 to November 2017 at Thoracic Surgery Department in Shanghai Pulmonary Hospital, Shanghai, China. All data were collected from patient computerized records and medical notes. Written informed consent was obtained from the patient for the surgical procedure and possible use of the data and/or any accompanying images or videos in scientific publication. A copy of the written consent is available for review by the Editors-in-Chief of this journal. The study was approved by the hospital’s Institutional Review Board (IRB).
Inclusion criteria
Preoperative discussion is held by our team to select patients most suitable for SVATS lobectomy. We select cases with:
- Lung cancer with T1, T2 status of tumor <7 cm with lesion suspicious for malignancy in positron emission tomography (PET) scan and located within the vicinity of the lobe with no possibility of wedge resection nor segmentectomy;
- N0 status for tumor;
- Localized infectious lung disease;
- Forced expiratory volume in 1 s (FEV1) and diffusing capacity of the lungs for carbon monoxide (DLCO) >40% postoperative predicted.
Exclusion criteria
Exclusion criteria were the following: central masses, enlarged lymph nodes with confirmed N1 or N2 disease, chest wall involvement, previous thoracic surgery, cardiomegaly and BMI >30. We also excluded cases underwent SVATS resections of bilateral pulmonary or concomitant mediastinal and pulmonary lesions.
All patients were subjected to the followings
Preoperative full history taking and clinical examination. Preoperative full laboratory investigations, computed tomography (CT) scans of the chest, PET scans, pulmonary function tests, flexible bronchoscopy, histological diagnostic tests if possible and echocardiogram. Intraoperative histology was carried out in all cases using frozen tissue sections followed by detailed confirmatory pathological report postoperatively. Postoperative follow up of our patients ranged from 3 months to 3 years.
Perioperative demographic data, intraoperative and postoperative findings and complications were collected and analyzed. The patients were divided into four groups chronologically according to date of operation. Group 1, group 2, group 3 and group 4 comprise 1st 100 cases, 2nd 100 cases, 3rd 100 cases and 4th 138 cases respectively. All data was analyzed for all patients as a whole then the most important findings were analyzed between the four groups to study the change of different variables with progress of the learning curve.
General operative set up
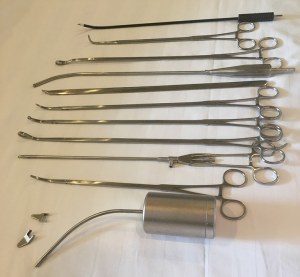
Only one monitor is used and placed cranially above the head of the patient, the surgeon and the scrub nurse stand on the abdominal side of the patient with the assistant stands on the opposite side. All SVATS lobectomies are performed with a 10 mm, 30-degree angled HD video-thoracoscope. Usual VATS instruments plus self-made dedicated instruments specially designed for SVATS which is longer, harder with more angled ends (Shanghai Medical Instruments Group Ltd.) are used during the operations (Figure 1).
Anesthesia and positioning of the patients
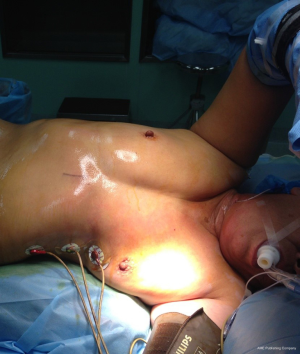
All cases are performed under general anesthesia with double lumen tube (single lung ventilation). The patient is placed in the lateral decubitus position with a backward inclination of 30°. The operative field is sterilized widely, to allow conversion to uniportal, multiple port approaches or thoracotomy once needed (Figure 2).
Surgical technique
A 3–4 cm longitudinal incision is made extending from xiphisternal junction to 1 cm below xiphoid process (Figure 2). The subcutaneous tissue is dissected then the rectus abdominis muscle is exposed and its fibers dissected longitudinally to expose the xiphoid process that is completely resected to provide a widened operative access. By index finger dissection, a retrosternal tunnel is created above the diaphragm with moving of the index finger more toward the operated side in a trial to open the pleura bluntly. A wound protector (Chinese Manufacture wound protector, Changzhou, China) is inserted giving more space for the camera and instruments.
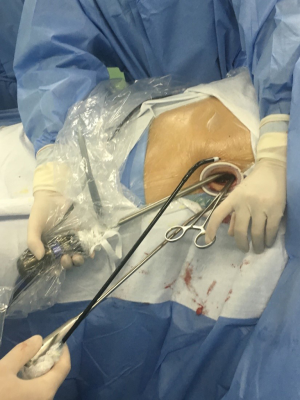
A 10-mm, 30° video camera is placed on the caudal side of the incision with the assistant holding the camera and pressing the camera slightly against the lower costal margin using it as a fulcrum for the camera. VATS instruments are placed from the cranial side of the incision (Figure 3).
The pleura of the operated side is opened under thoracoscopic visualization and the pericardiophrenic fat is removed which facilitates the passage of instruments and staplers. The pleural cavity is evaluated for unexpected pathology and the site of the lesion is ensured. The interlobar fissural status is assessed upon which approach of lobectomy is determined.
The lung is retracted using specially designed long curved lung grasper held by the other hand of the assistant and dissection is carried out by the surgeon using a suction instrument in the left hand and a laparoscopic hook, long electrocautery or 5 mm LigaSure blunt 37 mm tip (Covidien Ltd., Dublin, Ireland) in the right hand.
As general, we follow the order of stapling the artery first followed by vein then bronchus. If the fissure is well developed, we try to open it early to delineate the vascular anatomy, but if not well developed, we leave it as the last step or follow the fissureless technique by stapling the interlobar arteries and fissure last.
The vessels, bronchus and fissures are exposed and divided with appropriate strait or articulated endostaplers. In some cases, a curved-tip stapler technology is used to facilitate the passage around the structures. To transect minor pulmonary arteries, we use proximal and distal silk ligature or polymer clips (Click’a V® Endoscopic Polymer Clip Appliers 45°, Grena Ltd., Brentford, England) then resect in between with LigaSure. A specimen pouch Endo Catch bag 10 mm (Covidien Ltd.) is used to remove the specimen, with prior removal of the wound protector.
Systemic lymph node dissection is performed from at least three N2 stations according to the IASLC/Mountain classification. We prefer usage of energy devices in lymph node dissection which helps with extracapsular dissection along with optimum hemostasis.
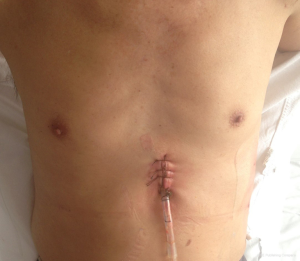
After surgery, a 28 F pleural drainage tube is inserted at the inferior end of the incision passing laterally to the thoracic apex, and then connected to a drainage bottle (Figure 4). A deep venous puncture catheter is inserted at the 8th intercostal space on lateral chest wall then connected to a drainage bag as a basal drain. The incision is closed in layers. All patients are extubated on table.
Postoperative care
Postoperatively, all patients are shifted to the intensive care ward then further transferred to the normal ward if there is no complication or drainage more than 500 mL in the first 6 hours. Postoperative pain is managed using a patient-controlled analgesia pump, as required, with sufentanil citrate 1 mL: 50 µg, and regular medication with flurbiprofen 50 mg every 6 h, alternating with paracetamol 1 g every 6 h. We follow early mobilization policy and chest physiotherapy. The tube is removed when there is no air-leakage and fluid drainage less than 300 cc in 24 hours. Patients are usually discharged one day after tube removal and seen 2 weeks, 1 month, 3 months then every 6 months intervals in the outpatient clinic.
Statistical analysis
Statistical analysis was carried out via Statistical Package for Social Science (SPSS) version 20 program on Windows 7. Qualitative data were represented by number and percent. Quantitative data were represented by mean ± standard deviation (mean ± SD) for normally distributed data, while non-normally distributed and discrete data were described by median, minimum and maximum. Kolmogorov-Smirnov test was used to test normality of quantitative data. Kruskal-Wallis test and Mann-Whitney test were used to compare non-normally distributed variables. One-way ANOVA was used to compare between means in the four different groups. Chi square test was used to compare qualitative data. Results were considered statistically significant if P value is less than or equal to 0.05.
Results
Patients characteristics
The study included 438 patients, 221 males (50.5%) and 217 females (49.5%) with mean age of 60.7±9.2 (range, 32–82) years. Lesion diameter mean size was 24.7±11.9 mm (range, 8–80 mm) with 2 reported cases with lesions diameter of 80 mm which was larger than the preoperative CT measured size. We didn’t exclude them as they didn’t affect the surgical technique or results.
Right upper lobectomy was the most common performed procedure (224 cases, 51.1%), right upper and middle bilobectomy were performed in 6 cases (1.4%) and right middle and lower bilobectomy was performed once (0.2%). Rest of the cases underwent other types of single lobectomies.
Our series included both malignant (362 cases, 82.6%) and benign (76 cases, 17.4%) pathologies. Infiltrating adenocarcinoma was the most common malignant pathology (261 cases, 59.6%) while tuberculous cavitary lesions (21 cases, 4.8%) was the most common benign pathology.
Operative and postoperative findings and the significance of their difference between the 4 groups are described in Table 1.
Table 1
| Findings | Group 1 (n=100) | Group 2 (n=100) | Group 3 (n=100) | Group 4 (n=138) | P value |
|---|---|---|---|---|---|
| Operative time*** | 2.82±0.82 | 2.38±0.72 | 1.98±0.6 | 1.62±0.6 | <0.001* |
| Intraoperative blood loss | 100** [40–600] | 100** [0–200] | 50** [0–600] | 50** [0–500] | <0.001* |
| Number of dissected LN stations | 4** [3–6] | 5** [3–7] | 5** [3–7] | 5** [2–9] | <0.001* |
| Number of dissected LN | 13** [7–22] | 14** [6–20] | 12** [6–18] | 12** [4–24] | <0.001* |
| Postoperative stay | 4** [2–13] | 4** [2–11] | 4** [2–14] | 3** [1–11] | 0.001* |
| Postoperative drainage | 250 [10–850] | 300 [0–800] | 200 [0–700] | 200 [0–800] | >0.05 |
Data are presented as mean ± SD or median [range]. *, P value less than 0.05 is considered statistically significant. **, (I) intraoperative blood loss decreased with statistical significance in groups 2, 3 and 4 in relation to group 1; (II) number of dissected LN stations increased with statistical significance in groups 2, 3 and 4 in relation to group 1; (III) number of dissected LN increased with statistical significance in groups 3 and 4 in relation to group 1. Also, increased with statistical significance in group 4 in relation to group 2; (IV) postoperative stay decreased with statistical significance in group 4 in relation to groups 1, 2 and 3. ***, operative time reduced progressively between all groups with statistically significant difference. LN, lymph node.
- Operative time reduced progressively between all groups with statistically significant difference.
- Intraoperative blood loss decreased with statistical significance in groups 2, 3 and 4 in relation to group 1.
- Number of dissected lymph node (LN) stations increased with statistical significance in groups 2, 3 and 4 in relation to group 1.
- Number of dissected LN increased with statistical significance in groups 3 and 4 in relation to group 1. Also, increased with statistical significance in group 4 in relation to group 2.
- Postoperative stay decreased with statistical significance in group 4 in relation to groups 1, 2 and 3.
- There was no statistical significance between all groups regarding postoperative drainage (P>0.05).
Operative and postoperative complications in the 4 groups are shown in Table 2. Postoperative complications occurred in 28 cases in an overlapping form (sometimes, one patient had more than one complication). Prolonged air leak (more than 7 days) was the most common complication (15 cases, 3.4%) followed by arrhythmia (14 cases, 3.2%). We had only one case of accidental cerebral infarction followed by mortality. Only one case had postoperative bleeding and reoperation through thoracotomy. There was no statistical significance between all groups regarding rate of conversion nor complications (P>0.05).
Table 2
| Variables | Group 1 (n=100) | Group 2 (n=100) | Group 3 (n=100) | Group 4 (n=138) |
|---|---|---|---|---|
| Conversion | 10 (10%) | 4 (4%) | 1 (1%) | 1 (0.7%) |
| Uniportal intercostal approach | 6 (6%) | 1 (1%) | 1 (1%) | 1 (0.7%) |
| Bleeding | 1 | |||
| Adhesions | 4 | 1 | ||
| Technical difficulty | 2 | 1 | ||
| Thoracotomy | 4 (4) | 3 (3%) | 0 (0%) | 0 (0%) |
| Bleeding | 3 | |||
| Adhesions | 1 | 3 | ||
| Postoperative complications | ||||
| Prolonged air leak | 4 (4%) | 2 (2%) | 6 (6%) | 3 (2.2%) |
| Arrhythmia | 4 (4%) | 3 (3%) | 2 (2%) | 5 (3.6%) |
| Thoracic hematoma | 1 (1%) | 1 (1%) | 1 (0.7%) | |
| Wound infection | 1 (1%) | 2 (2%) | ||
| Empyema | 1 (1%) | |||
| Pneumothorax with reinsertion of ICD | 1 (1%) | |||
| Pulmonary embolism | 1 (0.7%) | |||
| Post-operative bleeding/reoperation | 1 (0.7%) | |||
| Cerebral infarction/mortality | 1 (1%) |
ICD, intercostal chest drain.
Postoperative follow up resulted in discovery of metastasis in 7 patients (1.6%), described in Table 3. No local recurrence was reported.
Table 3
| Metastasis | Group 1 (n=100) | Group 2 (n=100) | Group 3 (n=100) | Group 4 (n=138) | Total (n=438) |
|---|---|---|---|---|---|
| Number of cases | 3 (3%) | 2 (2%) | 2 (2%) | 0 | 7 (1.6%) |
| Type of metastasis | Pleural [2]; cerebral [1] | Pleural [1]; distant [1] | Distant [2] | – | Pleural [3]; cerebral [1]; distant [3] |
| Interval between surgery and appearance of metastasis (months) | 9–33 | 6–20 | 9–12 | – | 6–33 |
Discussion
Subxiphoid approach has been widely used in thoracic surgery for various indications such as thymectomy, metastasectomy., and pneumothorax (11-13). Role of subxiphoid approach in major lung resection with its early learning curve has been addressed through some case series (9,14). In this article, we present our up to date experience for SVATS lobectomy from the surgical point of view with a trial to show the evolution of the technique and change in the results through the progress of learning curve.
This study included 438 patients who underwent uniportal SVATS lobectomy from September 2014 to November 2017 with exclusion of cases underwent SVATS resections of bilateral pulmonary or concomitant mediastinal and pulmonary lesions (10). We included cases with lesion size less than 7 cm diameter. Actually, we started our subxiphoid experience by selecting lesions of less than 5 cm diameter (9) which was increased with gaining experience (after 200 cases) to meet the same size criteria of intercostal uniportal approach. In group 4, we met two cases of tumor size of 8 cm with no invasion of visceral pleura. The size of the lesions in these two cases was 1 cm larger than the preoperative CT assessment. However, we didn’t exclude them as that size didn’t increase the difficulty of lobectomy or extraction of the resected lobe.
The patient is positioned in lateral decubitus position. The 30° posterior inclination helps to move the lung backwards giving clearer view of the hilum, more spacious space for instruments with less interference between the instruments and the mediastinum specially with the heart on the left side. Moreover, it gives the surgeon’s hand more space to move and handle the instruments freely. We noticed also that 30° backward inclination makes better ergonomics for the surgeon’s wrist as it makes hilar and fissural structure come at right angle with the long curved tipped instruments which facilitates dissection with less effort.
Longitudinal subxiphoid incision is currently made routinely in almost all cases regardless the degree of infrasternal angle. We previously operated via a horizontal incision if the infrasternal angle was ≥70° or via a longitudinal incision if the infrasternal angle was <70° (15). With time, we have thought that a longitudinal incision with its upper end at the xiphisternal junction makes the working port nearer to the hilum. In addition, the longitudinal incision goes with the same underlying dissected longitudinal planes of subcutaneous tissue, rectus muscle fibers and xiphoid process minimizing the degree of tissue trauma and giving an even and wider retraction by the wound retractor. As the incision is totally above the diaphragm, we haven’t had any cases of abdominal herniation.
Previous experience in uniportal intercostal VATS lobectomy is a very helpful for the surgeon specially at the early learning curve. It eases surgeon’s hands coordination in such technique with difficult angles. Also, presence of experienced assistant is vital for keeping the maneuver smooth and safe. The assistant’s movement should be fully coordinated with the surgeon to view difficult angles specially at apical arterial trunks and posterior mediastinal structures. The assistant should go back by the camera till the wound retractor each time the surgeon introduces new instrument to the field to make that instrument under vision to avoid overseen accidental injury of the heart specially on the left side.
With ongoing experience, special self-made instruments for SVATS were designed to overcome the relatively longer track and different axis of subxiphoid approach than the intercostal one. Those instruments are longer, harder with more curved tips, including lung graspers, dissectors and electrocautery blades. They actually have helped in lesser instruments interference, easy and comfortable exposure, dissection and cauterization of tissues. Also, use of curved tips staplers facilitates easier division of different structures during the procedure.
Order of dissection and dividing of pulmonary vessels and bronchi had been followed classically by starting with the artery, vein then the bronchus specially at the start of our learning curve associated with precise selection of strait forward cases with classic anatomy and minimal or no adhesions. With experience, we started to accept operating more challenging cases with more advanced adhesions or anatomical variations required us to follow the role of “whatever easy comes first”, we no longer stick to a specific order in all cases including sometimes the fissureless technique following the same way as in intercostal approach.
In all indicated cases, systemic lymph node dissection of at least three N2 lymph nodes stations is performed according to the IASLC/Mountain classification. Subcarinal lymph node sampling or dissection is routinely done. The first 100 cases experience were enough to show statistical significance increase in the number of dissected LN stations starting from group 2 with steady learning curve through groups 3 and 4. Number of dissected lymph nodes increased significantly in group 3 and 4 in relation to group 1. Actually, we started our experience by lymph node sampling with some difficulty in lymph node dissection specially with posteriorly located subcarinal lymph nodes. With getting more experience and adjustment of special instrument set, we started to get more lymph nodes.
Cardiac compression and arrhythmia are challenges on left sided operations. Improved handling skills, backward tilting of the patient, longer and specially curved instruments making the concave edge of the instruments toward the heart during the operation have enabled us to make better retraction and dissection of pulmonary structures with lesser compression on the heart. We adopt the longitudinal midline incision in our cases even in the left sided ones and we didn’t find statistically significant change in the rate of arrhythmia between all groups. So, we haven’t found any more importance to make left sided subcostal incision as a tool to decrease cardiac arrhythmia (10), but actually it is still an option according to surgeon’s preference.
Sometimes in case of bleeding, extensive adhesions or technical difficulty, we convert to intercostal approach through adding of extra intercostal incision at the 5th intercostal space between anterior and mid axillary lines which is then converted to anterior thoracotomy if needed. The rate of conversion has decreased from 10 cases in group 1 to only one case in group 3 and group 4. In group 1, the main cause of conversion to intercostal approach was adhesions (4 cases) and technical difficulty on left side (2 cases), and the main cause of conversion to thoracotomy was bleeding (3 cases) and adhesions (1 case). With experience and specially designed instruments, we have been able to deal with bleeding and cases with more extensive adhesions through SVATS, only one case was converted to intercostal approach in group 3 due to adhesions and another one in group 4 due to bleeding without the need to thoracotomy.
Intraoperative blood loss significantly decreased starting from group 2 going through groups 3 and 4 in relation to group 1. That has been attributed to occurrence of 3 cases of intraoperative bleeding in group 1 at the beginning of our experience with relatively prolonged unsuccessful trials to stop bleeding which led to significant blood loss before conversion to thoracotomy. With better instrumentation and experience, incidence of accidental intraoperative bleeding has decreased with more ability to faster control through SVATS if occurs. According to such finding, we consider SVATS as a safe approach for lobectomy.
We had only one case of mortality in 75 years old male with postoperative accidental coma and cerebral infarction with no response to specific treatment. The cause of death wasn’t related to a surgical cause. Only one case who underwent right upper lobectomy had postoperative bleeding and reoperation through thoracotomy.
Postoperative metastasis was discovered on follow up in only 7 patients (1.6%) with interval of 6 to 33 months between surgery and discovered metastasis. No local recurrence was reported. That may denote the effective oncological clearance of SVATS approach. Off course further studies with longer follow up are needed to confirm that.
As in other approaches of VATS lobectomy, operative time has been going inversely with the increasing experience and number of cases (16-18). However, learning curve for SVATS lobectomy may be harder and more prolonged because of the different caudal-cranial view and longer distance to the hilum causing more difficult handling of instruments (14). The operation time in our series has significantly decreased after the first 100 cases (group 1), continuing to go down progressively through all groups. We see that is the net result of evolution of all components of the learning curve in the form of; getting the key to the best ergonomics for positioning and general set, being more familiar with the view, optimized instruments, easier handling for lobectomy and LN dissection and more effective and faster dealing with intraoperative difficulties.
Limitation of the study
Postoperative follow up of our patients ranged from 3 months to 3 years. From our point of view, longer uniform follow up is required for all patients to investigate long term effectiveness of SVATS lobectomy. Also, we consider that presence of selection criteria for patient who will undergo SVATS lobectomy is still a restricting point to show its generalizability. So, we believe that the next step should be a randomized trial through which the SVATS lobectomy is operated without specific inclusion criteria different from that of intercostal approach.
Conclusions
Adjustment of some surgical steps and instrumentation along with developing experience have helped SVATS lobectomy to be easier, safer and faster. Complete oncological clearance and adequate lymph nodes dissection is feasible in cases with malignant pathology.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Crisci and Luca Bertolaccini) for the series “Surgical Approaches to VATS Lobectomy: Meet the Experts” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.10.05). The series “Surgical Approaches to VATS Lobectomy: Meet the Experts” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the hospital’s Institutional Review Board (K17-160). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim D, Kim HK, Choi YS, et al. Is video-assisted thoracic surgery lobectomy in benign disease practical and effective? J Thorac Dis 2014;6:1225-9. [PubMed]
- Mazzella A, Olland A, Garelli E, et al. Video-assisted thoracoscopic surgery is a safe option for benign lung diseases requiring lobectomy. Surg Endosc 2017;31:1250-6. [Crossref] [PubMed]
- Hanna WC, de Valence M, Atenafu EG, et al. Is video-assisted lobectomy for non-small-cell lung cancer oncologically equivalent to open lobectomy? Eur J Cardiothorac Surg 2013;43:1121-5. [Crossref] [PubMed]
- Zhang Z, Zhang Y, Feng H, et al. Is video-assisted thoracic surgery lobectomy better than thoracotomy for early-stage non-small-cell lung cancer? A systematic review and meta-analysis. Eur J Cardiothorac Surg 2013;44:407-14. [Crossref] [PubMed]
- Ismail NA, Elsaegh M, Dunning J. Novel Techniques in Video-assisted Thoracic Surgery (VATS) Lobectomy. Surg Technol Int 2015;26:206-9. [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Liu CC, Wang BY, Shih CS, et al. Subxiphoid single-incision thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg 2014;148:3250-1. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Lei J, et al. Subxiphoid uniportal video-assisted thoracoscopic middle lobectomy and anterior anatomic segmentectomy (S3). J Thorac Dis 2016;8:540-3. [Crossref] [PubMed]
- Hernandez-Arenas LA, Lin L, Yang Y, et al. Initial experience in uniportal subxiphoid video-assisted thoracoscopic surgery for major lung resections. Eur J Cardiothorac Surg 2016;50:1060-6. [Crossref] [PubMed]
- Song N, Zhao DP, Jiang L, et al. Subxiphoid uniportal video-assisted thoracoscopic surgery (VATS) for lobectomy: a report of 105 cases. J Thorac Dis 2016;8:S251-7. [PubMed]
- Weaver H, Ali JM, Jiang L, et al. Uniportal subxiphoid video-assisted thoracoscopic approach for thymectomy: a case series. J Vis Surg 2017;3:169. [Crossref] [PubMed]
- Liu CC, Wang BY, Shih CS, et al. Subxyphoid single-incision thoracoscopic pulmonary metastasectomy. Thorac Cancer 2015;6:230-2. [Crossref] [PubMed]
- Liu CY, Lin CS, Liu CC. Subxiphoid single-incision thoracoscopic surgery for bilateral primary spontaneous pneumothorax. Wideochir Inne Tech Maloinwazyjne 2015;10:125-8. [Crossref] [PubMed]
- Hernandez-Arenas LA, Guido W, Jiang L. Learning curve and subxiphoid lung resections most common technical issues. J Vis Surg 2016;2:117. [Crossref] [PubMed]
- Aresu G, Wu L, Lin L, et al. The Shanghai Pulmonary Hospital subxiphoid approach for lobectomies. J Vis Surg 2016;2:135. [Crossref] [PubMed]
- Bedetti B, Bertolaccini L, Solli P, et al. Learning curve and established phase for uniportal VATS lobectomies: the Papworth experience. J Thorac Dis 2017;9:138-42. [Crossref] [PubMed]
- Li X, Wang J, Ferguson MK. Competence versus mastery: the time course for developing proficiency in video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2014;147:1150-4. [Crossref] [PubMed]
- Cheng YJ. The learning curve of the three-port two-instrument complete thoracoscopic lobectomy for lung cancer-A feasible technique worthy of popularization. Asian J Surg 2015;38:150-4. [Crossref] [PubMed]
Cite this article as: Abdellateef A, Yang C, Chen J, Qu J, Huang J, Jiang L. Subxiphoid uniportal video assisted thoracoscopic surgery lobectomy, evolution of the technique and progress of learning curve. Shanghai Chest 2018;2:88.


