
Surgical trials in mesothelioma—past, present and future
Introduction
Surgery has been performed for malignant pleural mesothelioma (MPM) for over 50 years, yet the role of radical surgery remains a topic of great controversy (1). Whilst surgery has been undertaken using both open and thoracoscopic approaches, the majority of evidence has, until the last few years, been based on highly selected cohort studies.
When considering the management of cancer, one must balance the risks of treatment versus the benefits, which can be expressed simply as length and quality of life. Until recently, published data of MPM surgery were reliant on uncontrolled case series or cohort studies, which have inherent limitations due to the inevitable selection bias towards younger and fitter patients with earlier stage disease, producing apparently acceptable outcomes. Unfortunately, many of these studies fail to acknowledge the effect of selection bias and ignore the difference between association and causative effects in judging outcomes. There may be instances where randomised controlled trials (RCTs) are not required in order to prove value: where, for example, cross-over studies demonstrate in the same patients definitive benefits following intervention compared to prior to the intervention. However, despite the best designed non-randomised studies using methods such as matched propensity scored analyses, there is no higher level of evidence than well designed, statistically powered RCTs. Whereas “before versus after” cross-over studies may yield evidence about the benefit of quality of life, evidence about a survival benefit can only be gained reliably from RCTs.
In the United Kingdom we are well-placed ton conduct RCTs of mesothelioma surgery. There is an increasing incidence of the disease within a state-funded healthcare system, with an increasing number of regional specialist mesothelioma multi-disciplinary team meetings. Patients are reluctant to seek treatment outside the National Health Service due to the expense and difficulty of access. We have been successful in the conduct of a strong portfolio of trials, including those examining the role of surgery, complemented colleagues in other countries. Two RCTs, conducted in the UK since 2003 and analyzing the role of surgery in MPM, have been published and two further RCTs are currently recruiting.
When considering the effects of surgery for MPM, it is critical to ensure that the definitions of different surgical operations are consistent. The Staging and Prognostic Factors Committee of the International Association for the Study of Lung Cancer conducted an international consensus exercise in order to unify definitions of surgery (2):
- Extrapleural pneumonectomy (EPP): en bloc resection of the parietal pleura, pericardium, diaphragm, lung and visceral pleura;
- Extended pleurectomy/decortication (EP/D): parietal and visceral pleurectomy, with the goal of complete macroscopic resection (CMR), with resection of the diaphragm and/or pericardium as required;
- Pleurectomy/decortication (P/D): parietal and visceral pleurectomy to remove all gross tumour, but without resection of the diaphragm or pericardium;
- Partial pleurectomy (PP): partial removal of parietal and/or visceral pleura for diagnostic or palliative purposes but leaving gross tumour behind. This may be performed by video assisted thoracoscopic surgery (VATS) or via a thoracotomy.
Essential in the consideration of the end point of surgery is the concept of CMR (3). It is recognized that the volume of residual tumour after surgery is a prognostic factor (4). Given that conventional clear microscopic margins cannot be achieved in patients with MPM, the most “radical” objective of MPM surgery is gaining a CMR, the primary aim of which is to increase the length of life. Hence the term “radical” must be used with caution, in view of the fact that conventional clear resection margins cannot be gained. However, for palliative surgery, the objective of minimizing the volume of residual disease is secondary to the aim of improving symptoms through optimizing chest wall and pulmonary mechanics.
Video assisted thoracoscopic surgery partial pleurectomy (VATS-PP)
The least invasive option of surgical resection is VATS-PP, which was investigated in the MesoVATS trial (5). MesoVATS was an open label, RCT which compared efficacy of VATS-PP with talc pleurodesis in 196 randomised patients. Eighty eight patients had talc pleurodesis and 87 underwent VATS-PP.
VATS-PP is usually performed through 2–3 ports and a costal, parietal and possibly upper mediastinal pleurectomy is performed. The diaphragm and pericardium are not resected. Through a VATS approach, a total visceral pleurectomy is difficult and usually incomplete. Data from the MesoVATS trial published in the online supplement suggested that, at best, 75% of the tumour was resected, resulting in an R2 resection margin (5). It is accepted that PP is a palliative procedure with the primary aim of improving quality of life, rather than survival.
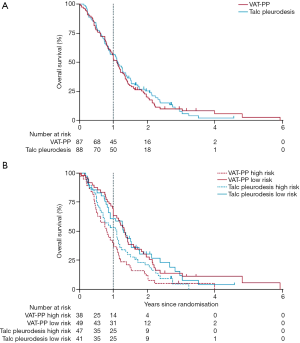
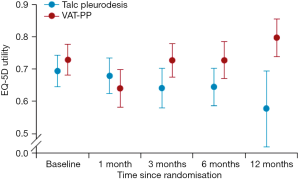
The MesoVATS trial showed no improvement in overall survival with VATS-PP (Figure 1A). However patients with a high prognostic risk, as defined by a modified EORTC Prognostic Group (6), had a significantly worse survival after VATS-PP, compared to those who had no surgery (Figure 1B). The surgical group had a modest improvement in quality of life, but at the expense of surgical complications, including a longer median hospital stay and greater cost when compared with talc pleurodesis, without prolongation of life. Improvement in EQ-5D scores in the VATS-PP group suggests that this treatment might have a role for low risk patients likely to survive over 6 months (Figure 2). This subcategory of patients may therefore benefit from further research (7).
Developing the theme of VATS-PP, the MesoVATS, study team have recently opened another RCT to accrual in the UK. Recognizing, firstly, the growing role of intrapleural catheters (IPCs) in the management of a trapped lung and, secondly, the potential for greater symptomatic benefit from VATS-PP in patients with a trapped lung, the MesoTRAP trial will investigate if outcomes are better in this patient group (8). Given that the incidence of trapped lung in MPM is unknown and considering that the patient related factors affecting willingness of eligible patients to enroll in such a study are unknown, initially a feasibility trial is being performed to test recruitment rates. Thereafter, if the feasibility criteria of randomizing 38 patients in 18 months are met, MesoTRAP aims to expand to a Phase III RCT fully powered to detect differences in primary outcomes of length and quality of life. Rather than a VATS-PD (which would imply resection of all macroscopic tumour, according to the IASLC consensus definitions), the VATS-PP proposed in MesoTRAP includes sufficient visceral pleurectomy to regain lung expansion, with or without an accompanying parietal pleurectomy. Patient-related study outcomes include visual analogue scale scores for dyspnoea and chest pain and quality of life at baseline and post intervention (8). To date, 501 patients have been screened for MesoTRAP, and 67 (13%) have been diagnosed with trapped lung. Of these, 45 did not meet the eligibility criteria for the study and 14 declined participation. Eight patients have been randomised.
EPP
At the other end of the surgical spectrum is EPP, where, with careful patient selection, CMR is expected. EPP was first described in a mid-20th century case series as a treatment for tuberculosis (9). The first series of EPP in MPM patients was published in 1976, with cases dating back to the 1950’s. Butchart reported a perioperative mortality rate of 31%, which has improved in modern case series to between 2.2% and 7% (10).
The mesothelioma and radical surgery (MARS) trial was a multicentre RCT of induction chemotherapy, EPP, adjuvant hemithorax radiotherapy, versus no surgery (11). Rather than a Phase III trial statistically powered with regard to primary outcomes of quality of life or survival, it was designed as a feasibility randomised study aimed to test if 50 patients, all having completed platinum based chemotherapy, could be randomised in the UK between EPP or no surgery. Patients randomised in the trial were highly selected, with prior staging mediastinoscopy and a two-stage consent process (before and after induction chemotherapy). The primary outcome was to determine the feasibility of randomizing 50 patients in one year, with secondary outcomes of survival and quality of life.
Recruiting from 12 UK oncology centres, it took three years to reach 50 randomizations, rather than the target of one year. During the three-year accrual period, 257 patients were screened, 112 were registered and received induction chemotherapy. Fifty patients proceeded to randomised between EPP or no surgery.
Of the 112 patients recruited, 33 patients suffered disease progression prior to randomisation, 19 patients declined randomisation and 5 patients become medically inoperable. Of the 24 randomised to surgery, 16 patients completed EPP: EPP was not performed in 3 patients due to refusal post randomisation and to clinical decision in 2 and EPP was abandoned in 3 patients. Adjuvant radical radiotherapy was performed in only 8 of the 16 patients completing EPP. Of the 26 patients randomised to no surgery, 3 received EPP and 3 non-EPP surgery outside of the trial framework. Thus there was poor compliance within both randomised arms of the trial protocol. In addition, the chemotherapy regimens varied, due to the pemetrexed-platinum doublet combination not yet being established as the standard. Secondary outcome data showed a large variation between the various trial centres reflected in the high perioperative mortality (12).
Although, as a feasibility study, MARS was not being statistically powered for overall survival as an outcome, median survival in the EPP group was 14.4 months, as opposed to 19.5 months in the no-EPP group (P=0.082, Figure 3). Median quality of life scores were lower in the EPP group and scores stayed below the no surgery group for the duration of the follow up period, although differences were not statistically significant (Figure 4).
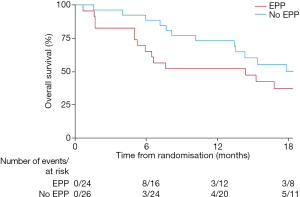
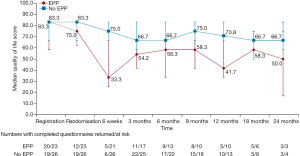
The results of the MARS trial have been the subject to much debate. The study investigators acknowledged the lack of feasibility of performing a fully powered RCT of EPP in the UK. However, although the survival and quality of life results are provocative, they are not statistically valid due to the small group sizes. Despite this, it is clear that EPP has fallen from favour, not just in the UK but around the world (13). Publications continue to appear presenting cohorts of highly selected patients undergoing EPP, but no group has launched a further RCT to examine the potential merits of surgery vs. no surgery. A study published after the MARS trial concluded that EPP caused significant deterioration in lung function and may lead to shortness of breath, negatively impacting quality of life (14). Several studies have reviewed the role of EPP and it has been proposed that the procedure did not improve overall survival compared to EP/D (15).
However, amongst these cohort studies, a promising new protocol of accelerated hemithoracic intensity-modulated radiation therapy followed by EPP has emerged. This regime, of 25 Gy of radiotherapy in 5 fractions over 1 week, given 6 days prior to EPP, has yielded a median survival for all patients as an intention-to-treat analysis of 36 months and encouraging results in patients with epithelial subtype (16). As with any non-randomised study, the degree of selection to the study is unknown and it remains unclear whether a RCT will test this protocol compared with systemic chemotherapy alone.
EP/D
EP/D involves resection of the pericardium and/or the diaphragm, in addition to the parietal pleurectomy and visceral decortication. Until the publication of the IASLC definitions consensus paper, reports of lung-sparing P/D operations used varying taxonomy, making comparison of outcomes between studies difficult. Although large cohort studies have been published comparing EP/D and EPP, selection bias affects their interpretation. Some surgeons have preferred EPP in early stage disease, preserving EP/D for more advanced stages, whereas others have performed the opposite (15). Similarly, selection bias affects the interpretation of survival rates in non-randomised cohort studies. However, the effects of EP/D on lung function and quality of life have been assessed in a number of cross-over cohort studies, where measurements after surgery were compared to those beforehand. However, the results of these studies do not provide sufficient justification to obviate the need for a randomised trial with quality of life as an important endpoint. Evidence from the MesoVATS trial suggested that incomplete macroscopic resection with VATS-PP did not prolong life, although there were trends towards a modest improvement in quality of life. The ‘more radical’ EPP, whilst gaining CMR, was associated with a trend towards worse quality of life and also had no clear survival benefit in the MARS feasibility trial. Hence the MARS-2 trial was established, with the hypothesis that EP/D results in better quality of life, by preserving the lung, but also better survival by achieving CMR, compared to no surgery.
The MARS-2 trial is accruing currently well in the UK. It is the first RCT analyzing the role of EP/D versus no surgery. Patients are randomised if there is no evidence of disease progression on CT following 2 cycles of standard of care chemotherapy. The feasibility phase of the trial is now complete (17). The trial is currently open in 22 regional medical oncology centres across the UK, and surgery for the first 50 randomizations was performed in one of two lead surgical centres, before three second wave surgical centres were opened. Recruitment is accruing to target, with 167 patients randomised out of a target of 328.
Another second randomised trial involving EP/D is the EORTC-1205 phase II randomised study of EP/D preceded by or followed by chemotherapy, for patients with early stage MPM (18). It is a trial examining the timing of chemotherapy but will not inform practice about the risks and potential benefits of surgery. Recruitment has been challenging and is not recruiting to target (18).
Discussion
There have been more uncontrolled case series than RCTs and analysis of the data has not definitively answered the questions raised regarding the role of surgery in MPM. RCTs remain a challenge to both design and conduct: however, trustworthy evidence is needed on which to base clinical practice (19). VATS-PP has no effect on overall survival and results in more complications and longer hospital stay compared to talc pleurodesis. VATS-PP may be of some quality of life benefit to patients that present with good prognostic features, but this is yet to be formally established. EPP is potentially harmful to patients. EP/D may result in lower perioperative mortality than EPP. EP/D may be of benefit in patients that are symptomatic at baseline and not be disadvantageous in minimally symptomatic patients: the MARS-2 trial aims to provide the clarity given by a prospective randomised trial (17).
The experience from the United Kingdom demonstrates that it is possible to conduct successful randomised trials examining the role of surgery in MPM. The two trials reported to date, MARS and MesoVATS, have shown that longstanding perceptions of benefits of surgery can and should be challenged within the framework of a well-designed prospective RCT. It is critical, when reading the literature, to understand the contribution of selection bias to apparently favourable results of surgery. However, multimodality trials which involve a randomisation between surgery, or not, are complex and difficult to undertake. Such trials may require comprehensive screening processes, referral between regional centres with the patient having to travel for specialist surgery. It is essential for the recruiting clinical teams to understand and convey to the patient that there is equipoise between the different arms of a randomised trial. This can be particularly challenging for trials involving surgery, where there may be the perception of considerable different impact between the treatment arms, which may not be so apparent in drug trials. Furthermore, it is essential for surgeons to recognize their conflicts of interest. It may, for example, be particularly challenging to conduct a randomised trial of surgery versus not in a healthcare system where the surgeon (and hospital) receives a fee per case. Perhaps the trials of mesothelioma surgery conducted within the United Kingdom’s National Health Service have been successful as there is no fee for case for the surgeon.
It is recognized in the trial design of MARS-2 that recruitment can be challenging and hence Qualitative Recruitment Intervention methods have been incorporated (20). This includes the interviews of staff involved in recruitment, recoding and analysis of consultations with patients, interview with patients and feedback to recruiting teams. This process is designed to seek and overcome barriers to recruitment, with the understanding of equipoise by staff and its conveyance to patients being a central part.
Another issue regarding trials of surgery is to ensure that there is strict quality control of the procedure. It is important to define and report carefully the individual surgical steps, ensuring that surgeons maintain the highest standards of surgical quality (21). The MARS-2 trial is attempting to optimize surgical quality with a number of steps: the surgical centre must have an established Mesothelioma Multi-Disciplinary Team and have a minimum of two surgeons participating in the trial. The participating surgeons must undergo accreditation by observing surgery at an established site and being observed by an established surgeon in their own institution. The results of surgery are documented by intraoperative video and photographs and audited against the surgical protocol to ensure adherence and that quality standards are met.
Several organizations have published guidelines for the management of mesothelioma, which are based on the available evidence. Hence it is interesting to note that there is differing opinion within these guidelines with regard to the role of surgery, as best informed by the RCTs.
The joint European Respiratory Society/European Society of Thoracic Surgeons guidelines for the management of mesothelioma recommend performing VATS P/D for symptomatic control in patients with trapped lung who are not going to benefit from talc pleurodesis and who have a predicted survival of over 6 months. It is stated that radical surgery in the form of EPP should only be performed as part of multimodality treatment in the context of clinical trials within specialist centres (22). However, there are no registered clinical trials investigating EPP in Europe at the current time. The more recent guidelines from the European Society of Medical Oncology (23) recommended that the indications for surgery resection were: for palliation of pleural effusions when chest tube drainage is not successful (Level II, Grade A); to be part of a multimodality treatment, preferably as part of a study (Level II, Grade A); to perform a macroscopic complete resection by means of P/D or EPP (Level III, Grade C).
The American Society for Clinical Oncology published clinical practice guidelines in January 2018 (24). These included the following statements. In selected patients with early-stage disease, it is strongly recommended that a maximal surgical cytoreduction (defined as EPP, EP/D or P/D) should be performed. Single modality surgery is insufficient and should be undertaken with multidisciplinary input. Patients with locally advanced (T4) disease or N2 (contralateral mediastinal or supraclavicular lymph node involvement) should undergo neoadjuvant treatment before consideration of maximal surgical cytoreduction surgery. Patients with sarcomatoid disease should not undergo maximal surgical cytoreduction. All the above were given a “strong” recommendation on the basis of the “intermediate” quality of evidence reviewed.
A month later, The British Thoracic Society also published guidelines for the investigation and management of mesothelioma (19). Three recommendations related to surgical resection were made: (I) do not offer VATS-PP over talc pleurodesis in MPM. (Grade A); (II) do not offer EPP (Grade B); (III) do not offer EP/D outside of a clinical trial (Grade D).
It is not clear why the authors of these guidelines, based on the same clinical evidence, differ in their opinions and recommendations with regards to the role of surgery in malignant mesothelioma, although this has been raised in the literature (25). It is possible that this is due to conflicts of interest. There may be differing levels of interpretation of trial data. For example, following the publication of the MARS feasibility trial, there was debate about the degree of validity of the quality of life and survival outcomes and whether there was over-interpretation of results (26,27).
Future trials
It remains to be seen whether a statistically powered RCT of EPP after induction radiotherapy will be possible, given the abandonment of this technique in all except a few centres worldwide. The results of MARS-2 will only be known in several years. If the overall primary outcomes are negative but subset analyses provocative, there may be the prospect of a further trial in patients, such as symptomatic patients with otherwise good prognostic factors. An interesting topic for future debate is whether surgical resection, if of neither benefit nor detriment in RCTs, provides a platform for more effective multimodality management including, for example, immune-oncology treatments, whereby the combination with surgery is better than without surgery.
Summary
In summary, the surgical resection of mesothelioma remains a controversial topic, despite the growing evidence from RCTs performed in the United Kingdom. Two trials, MARS and MesoVATS, have been published. Two trials, MARS-2 and MesoTRAP, are currently recruiting and will contribute to the evidence regarding whether surgical resection should, or should not, be performed.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (David Waller and Annabel Sharkey) for the series “Mesothelioma Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.11.06). The series “Mesothelioma Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lang-Lazdunski L. Surgery for malignant pleural mesothelioma: Why, when and what? Lung Cancer 2014;84:103-9. [Crossref] [PubMed]
- Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the International Association for the Study of Lung Cancer International Staging Committee and the International Mesothelioma Interest Group. J Thorac Oncol 2011;6:1304-12. [Crossref] [PubMed]
- Sugarbaker DJ. Macroscopic Complete Resection: The Goal of Primary Surgery in Multimodality Therapy for Pleural Mesothelioma. J Thorac Oncol 2006;1:175-6. [Crossref] [PubMed]
- Pass HI. Biomarkers and prognostic factors for mesothelioma. Ann Cardiothorac Surg 2012;1:449-56. [PubMed]
- Rintoul RC, Ritchie AJ, Edwards JG, et al. Randomised controlled trial of video-assisted thoracoscopic partial pleurectomy compared to talc pleurodesis in patients with suspected or confirmed malignant pleural mesothelioma: the MesoVATs trial. Lancet 2014;384:1118-27. [Crossref] [PubMed]
- Curran D, Sahmoud T, Therasse P, et al. Prognostic factors in patients with pleural mesothelioma:the European Organization for Research and Treatment of Cancer experience. J Clin Oncol 1998;16:145-52. [Crossref] [PubMed]
- Tsao AS. Video-Assisted Thoracoscopic Partial Pleurectomy for Malignant Pleural Mesothelioma, NEJM Journal Watch, Informing practice, July 2014.
- Rintoul R, Maskell NA, Edwards JG, et al. MesoTRAP: A feasibility study comparing video-assisted thoracoscopic partial pleurectomy/decortication with indwelling pleural catheter in patients with trapped lung due to malignant pleural mesothelioma designed to address recruitment and randomisation uncertainties and sample size requirements for a phase III trial. Lung Cancer 2018;115:S89. [Crossref]
- Milloy FJ, Langston HT. Pleuropneumonectomy in tuberculosis. Dis Chest 1958;34:593-601. [Crossref] [PubMed]
- Butchart EG, Ashcroft T, Barnsley WC, et al. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976;31:15-24. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Waller DA, Dawson AG. Randomized controlled trials in malignant pleural mesothelioma surgery-mistakes made and lessons learned. Ann Transl Med 2017;5:240. [Crossref] [PubMed]
- Azzouqa AG, Stevenson JP. The evolution of the diminishing role of extrapleural pneumonectomy in the surgical management of malignant pleural mesothelioma. Onco Targets Ther 2016;9:7247-52. [Crossref] [PubMed]
- Ploenes T, Osei-Agyemang T, Krohn A, et al. Changes in lung function after surgery for mesothelioma. Asian Cardiovasc Thorac Ann 2013;21:48-55. [Crossref] [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical-treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [Crossref] [PubMed]
- de Perrot M, Feld R, Leighl NB, et al. Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2016;151:468-73. [Crossref] [PubMed]
- MARS-2 Trialists. Lim E. Surgical selection in pleurectomy decortication for mesothelioma- an overview from screening and selection from MARS 2 pilot. J Thorac Oncol 2017;12:S1748. [Crossref]
- Raskin J, Surmont V, Hasan B, et al. EORTC 1205:Randomized Phase II Study Of Pleurectomy/Decortication Preceded Or Followed By Chemotherapy In Early Stage MPM. J Thorac Oncol 2018;13:S524. [Crossref]
- Woolhouse I, Bishop L, Darlison E, et al. BTS guideline for the investigation and management of malignant pleural mesothelioma. Thorax 2018;73:i1-i30. [Crossref] [PubMed]
- Donovan JL, Rooshenas L, Jepson M, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials 2016;17:283. [Crossref] [PubMed]
- Blencowe NS, Mills N, Cook JA, et al. Standardizing and monitoring the delivery of surgical interventions in randomized clinical trials. Br J Surg 2016;103:1377-84. [Crossref] [PubMed]
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Baas P, Fennell D, Kerr KM, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v31-9. [Crossref] [PubMed]
- Kindler HL, Ismaila N, Armato SG, et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1343-73. [Crossref] [PubMed]
- de Fonseka D, Slade M, Blyth KG, et al. An inconvenient truth concerning surgery for mesothelioma. J Clin Oncol 2018;36:2745-6. [Crossref] [PubMed]
- Weder W, Stahel RA, Baas B, et al. The MARS feasibility trial: conclusions not supported by data. Lancet Oncol 2011;12:1093-4. [Crossref] [PubMed]
- Bliss JM, Coombes G, Darlison L, et al. The MARS feasibility trial: conclusions not supported by data - Authors' reply. Lancet Oncol 2011;12:1094-5. [Crossref]
Cite this article as: Ajab S, Edwards JG. Surgical trials in mesothelioma—past, present and future. Shanghai Chest 2019;3:8.


