Diagnostic performance of glucose transporter-1 immunohistochemistry in malignant pleural mesothelioma: a meta-analysis
Introduction
Malignant pleural mesotheliomas (MPMs) are aggressive neoplasms with a dismal prognosis and a poor survival (1), whose incidence is increasing (2,3). Owing to the variety of histological patterns due to their celomatic origin, diagnosis can be challenging especially in face of bland cytomorphological features or small surgical specimens (4-6), resulting in diagnostic delay (7) and disagreement among pathologists. In this regard, both the US-Canadian Mesothelioma Reference Panel and the Group Mesopath reported a generalized lack of consensus about diagnostic criteria in up to 47% of members of the expert panel (8). Currently, two mesothelial markers and other two for different patterns represent the first step in the histologic pathway (9). However, according to the aforementioned issues and its poor prognosis (6), new tumor and molecular markers have rapidly gained interest. In this regard and on the attempt to clarify benign and malignant mesothelial features, immunohistochemical stainings have been proposed, though its role is still debated and controversial. Several markers have been reported to be used in this setting, such as p53, epithelial membrane antigen, Bcl-2 (10), insulin-like growth factor 2 messenger RNA binding protein-3 (IMP-3) (11) and desmin (12), but none of them present a significant diagnostic accuracy (DA) when considered alone. For these reasons, some speculative proposals have arisen, making some Authors to consider conventional H&E stainings more reliable than immunohistochemistry (13). Recently, immunohistochemical assay of glucose transporter-1 (GLUT-1) has been reported with promising results in diagnostic resolution; however, data are still to scanty and far from a general adoption. GLUT1, a transmembrane protein of the major facilitator superfamily (MFS), is widely distributed in normal tissues, such as erythrocyte membranes and brain tissues and its function is to expose alternately a binding site for glucose on membranes through a passive facilitated diffusion (14). Frequently upregulated during tumorigenesis, its expression usually relates to epithelial malignancies in a variety of organs (15-17).
Methods
Research strategy and study design
A PubMed Embase, Google Scholar research was carried out by three investigators from the authors’ panel in order to identify relevant articles published up to Aug 31, 2018. The MeSH keyword criteria were as follows: [“GLUT 1” (MeSH Terms) OR “glucose transporter 1” (All Fields)] AND [ “malignant pleural mesothelioma” (All Fields) OR “MPM” (All Fields) OR “pleural mesothelioma” (All Fields)] AND [“1980/01/01” (Date - Publication): “2018/08/31” (Date - Publication)]. All potential reports were reviewed, analysed and checked, if they fulfilled the following inclusion criteria: (I) diagnosis of primary pleural disease; (II) presence of a definitive histological diagnosis of MPM or reactive mesothelial diseases (RMDs); (III) GLUT-1 immunohistochemical assay on surgical specimens; (IV) proper definition of GLUT-1 staining and MPM avidity; (V) data clearly reported to contingency tables derivation for diagnostic evaluation; (VI) articles or papers or conferences’ papers written only in English. Letters to editor, reviews, states of art as far as case reports were excluded due to their poor statistical relevance. After definitive eligibility process, data were extracted by other two independent investigators by collecting the following information: authors, year of publication, number of enrolled patients for both cohorts (malignant and reactive diseases), absolute number (N) and percentage (%) of IHC+ GLUT-1 and ICH-GLUT-1 for each group and relative diagnostic value expressed as TP (true positive cases), FP (false positive cases), TN (true negative cases) and FN (false negative cases) occurrences.
Statistical analysis
The meta-analysis was conducted with Microsoft Excel 2016 (Microsoft®, Redmond, USA) and with IBM SPSS version 20.0 (IBM®, Segrate, MI, Italy). According to data extraction, 2×2 contingency tables (TP − TN vs. FP − FN) to determine sensitivity (Se), specificity (Spe), positive predictive value (PPV), negative predictive value (NPV), DA, disease prevalence (DP) and odds ratio (OR) were constructed. All data have been recorded as absolute values with their relative 95% confidence interval (95% CI). GLUT-1 immunostaining was evaluated according to cumulative positive rates into four braces: staining 0, absence of reactions; staining 1+, immunoreaction up to 10%; staining 2+, immunoreaction from 10% and 50% and staining 3+, immunoreaction greater than 50%. Se, Spe, PPV and NPV were calculated on the basis of the formulas as the ratio between TP/TP + FN, TN/TN + FP, TP/TP + FP and TN/TN + FN, respectively. Se, Spe and OR Forest plots were derived for each article and for cumulative occurrences, according to their weight percentage. Rough and 95% CI-adjusted DPs were analysed according to scattered plots with their R2 value. Finally, a summarized receiver operating curve (sROC) was derived for GLUT-1 IHC diagnostic performance.
Results
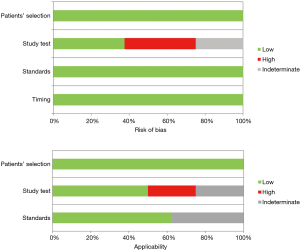
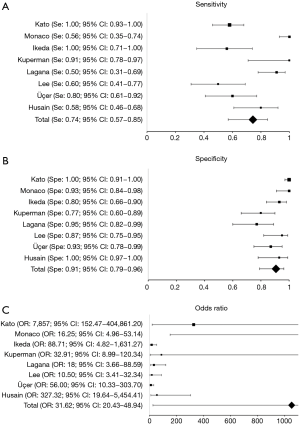
After a primary evaluation, 471 relevant articles were identified by two independent investigators for further analysis. Immediately, 451 were removed in accordance with their title or abstract and the remaining twenty underwent further full-text evaluation. A second-step analysis was brought throughout a careful assessment based on their study design and methods. Only eight eligible studies were identified as eligible (11,18-24) (Figure 1). In particular, 12 articles were excluded due to: (I) absence of peculiar data about IHC GLUT-1 assay details (seven articles); (II) form incompatibility (two review articles and an editorial) and finally; (III) inability to derive a 2×2 contingency tables due to lack of relevant data. Quality assessment of the eligible articles was carried out according to QUADAS-2 criteria (https://www.nice.org.uk/process/pmg6/resources/the-guidelines-manual-appendices-bi-2549703709/chapter/appendix-f-methodology-checklist-the-quadas-2-tool-for-studies-of-diagnostic-test-accuracy), as reported in Table 1. Potential sources of selection bias were identified in three articles, while in two an indeterminate risk was identified. Moreover, a high risk of study test-derived bias was noted in two reports and an indeterminate in five of them (two for study test and three for standards) (Figure 2). At the end of the abovementioned preliminary evaluation, 728 patients (297 MPM vs. 431 reactive pleural diseases) were enrolled. TP, FP, TN and FN cases were 215, 33, 398 and 82, respectively. In particular, 74.25% of MPM patients were GLUT-1 IHC+ (staining 1+: 18.98%, staining 2+: 28.29%, staining 3+: 26.98%, respectively) (Table 2) (Figures 3,4). Concerning with GLUT-1 diagnostic performance in MPM characterization, the pooled Se, Spe, PPV, NPV, DA and DP with their relative 95% CI were 0.74 (95% CI: 0.57–0.85), 0.91 (95% CI: 0.79–0.96), 0.87 (95% CI: 0.82–0.90), 0.83 (95% CI: 0.80–0.85), 0.84 (95% CI: 0.81–0.97) and 0.41 (95% CI: 0.37–0.44), respectively. Mean odds ratio was 31.62 (95% CI: 20.43–48.94) (Table 3). For pooled Se, Spe and OR, weighted-Forest plots were derived (Figure 5A,B,C) as far as a summarized-ROC curve, whose AUC was 0.61 (SE: 0.02, 95% CI: 0.47–0.60) (Figure 6).

Table 1
| Study | Risk of bias | Applicability | ||||||
|---|---|---|---|---|---|---|---|---|
| Patients’ selection | Study test | Standards | Timing | Patients’ selection | Study test | Standards | ||
| Kato et al. (19) | L | H | L | L | L | H | U | |
| Monaco et al. (18) | L | L | L | L | L | L | L | |
| Ikeda et al. (11) | L | U | L | L | L | L | L | |
| Kuperman et al. (22) | L | H | L | L | L | U | L | |
| Lagana et al. (23) | L | H | L | L | L | U | U | |
| Lee et al. (21) | L | U | L | L | L | H | L | |
| Üçer et al. (24) | L | L | L | L | L | L | U | |
| Husain et al. (20) | L | L | L | L | L | L | L | |
L, low; U, unclear; H, high.
Table 2
| Author | Year | Patients | MPM | RMD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MPM | RMD | Overall | Weight (%) | GLUT-1+, n (%) | GLUT-1−, n (%) | GLUT-1+, n (%) | GLUT-1−, n (%) | ||||
| Kato et al. (19) | 2007 | 48 | 40 | 88 | 12.09 | 48 (100.00) | 0 (0) | 0 (0) | 40 (100.00) | ||
| Monaco et al. (18) | 2011 | 27 | 70 | 97 | 13.32 | 15 (55.56) | 12 (44.44) | 5 (7.14) | 65 (92.86) | ||
| Ikeda et al. (11) | 2011 | 11 | 50 | 61 | 8.38 | 11 (100.00) | 0 (0) | 10 (20.00) | 40 (80.00) | ||
| Kuperman et al. (22) | 2011 | 43 | 35 | 78 | 10.71 | 39 (90.70) | 4 (9.30) | 8 (22.86) | 27 (77.14) | ||
| Lagana et al. (23) | 2012 | 30 | 38 | 68 | 9.34 | 15 (50.00) | 15 (50.00) | 2 (5.26) | 36 (94.74) | ||
| Lee et al. (21) | 2013 | 30 | 48 | 78 | 10.71 | 18 (60.00) | 12 (40.00) | 6 (12.50) | 42 (87.5) | ||
| Üçer et al. (24) | 2013 | 30 | 30 | 60 | 8.24 | 24 (80.00) | 6 (20.00) | 2 (6.67) | 28 (93.33) | ||
| Husain et al. (20) | 2014 | 78 | 120 | 198 | 27.20 | 45 (57.69) | 33 (42.31) | 0 (0) | 120 (100.00) | ||
MPM, malignant pleural mesothelioma; RMD, reactive mesothelial disease.
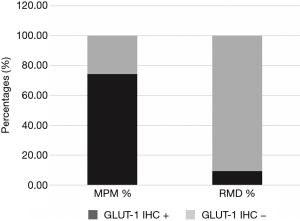
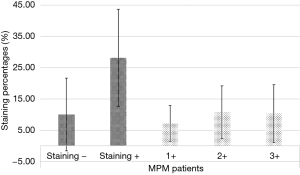
Table 3
| Author | MPM GLUT-1+ | ||||||
|---|---|---|---|---|---|---|---|
| Se (95% CI) | Spe (95% CI) | NPV (95% CI) | PPV (95% CI) | DA (95% CI) | DP (95% CI) | OR (95% CI) | |
| Kato et al. (19) | 1.00 (0.93–1.00) | 1.00 (0.91–1.00) | 1.00 | 1.00 | 1.00 | 0.54 (0.44–0.65) | 7,857 (152.47–404,861.20) |
| Monaco et al. (18) | 0.56 (0.35–0.74) | 0.93 (0.84–0.98) | 0.84 (0.78–0.89) | 0.75 (0.55–0.88) | 0.82 (0.73–0.89) | 0.28 (0.19–0.38) | 16.25 (4.96–53.14) |
| Ikeda et al. (11) | 1.00 (0.71–1.00) | 0.80 (0.66–0.90) | 1.00 | 0.52 (0.39–0.66) | 0.84 (0.72–0.92) | 0.18 (0.09–0.30) | 88.71 (4.82–1,631.27) |
| Kuperman et al. (22) | 0.91 (0.78–0.97) | 0.77 (0.60–0.89) | 0.87 (0.72–0.95) | 0.83 (0.72–0.90) | 0.85 (0.75–0.92) | 0.55 (0.43–0.66) | 32.91 (8.99–120.34) |
| Lagana et al. (23) | 0.50 (0.31–0.69) | 0.95 (0.82–0.99) | 0.71 (0.62–0.78) | 0.88 (0.85–0.97) | 0.75 (0.63–0.85) | 0.44 (0.32–0.57) | 18.00 (2.66–88.59) |
| Lee et al. (21) | 0.60 (0.41–0.77) | 0.87 (0.75–0.95) | 0.78 (0.69–0.85) | 0.75 (0.57–0.87) | 0.77 (0.66–0.86) | 0.38 (0.27–0.50) | 10.50 (3.41–32.34) |
| Üçer et al. (24) | 0.80 (0.61–0.92) | 0.93 (0.78–0.99) | 0.82 (0.69–0.91) | 0.92 (0.76–0.98) | 0.87 (0.75–0.94) | 0.50 (0.37–0.63) | 56.00 (10.33–303.70) |
| Husain et al. (20) | 0.58 (0.46–0.68) | 1.00 (0.97–1.00) | 0.78 (0.73–0.82) | 1.00 | 0.83 (0.77–0.88) | 0.39 (0.32–0.47) | 327.32 (19.64–5,454.41) |
| Total | 0.74 (0.57–0.85) | 0.91 (0.79–0.96) | 0.83 (0.80–0.85) | 0.87 (0.82–0.90) | 0.84 (0.81–0.87) | 0.41 (0.37–0.44) | 31.62 (20.43–48.94) |
Se, sensitivity; Spe, specificity; PPV, positive predictive value; NPV, negative predictive value; DA, diagnostic accuracy; DP, disease prevalence; OR, odds ratio; MPM, malignant pleural mesothelioma; GLUT-1, glucose transporter-1.
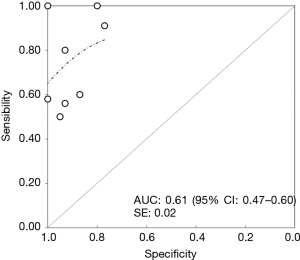
Discussion
MPM is a rare malignant disease mostly associated with asbestos exposure. Its incidence in Europe is about 20 per million inhabitants and it is increasing worldwide (3). A proper diagnosis between RMDs and malignant pleural proliferations can be challenging, as common features associated with neoplasms, such as high cellularity/mitoses or nuclear atypia, often are poorly reliable. For these reasons, immunohistochemical second-step assay has gained widely acceptance, especially for small surgical specimens, where an unequivocal surrounding tissue invasion may be absent. A number of markers have been proposed for conventional morphological diagnosis (12), but none of them has shown a high diagnostic accuracy to diagnose malignancy, except through the adoption of a panel of markers. Early results of FISH testing for p16 deletion were reported by Monaco et al. (18), who reported an overall Se of 59%, Spe of 100% and PPV rates of 100%. Authors, however, showed Se significantly augmented by considering only pleural malignancies rather than peritoneal ones (sensitivity: 70% vs. 51%). GLUT-1, a facilitative glucose transporter (25), is almost undetectable in all normal tissues or benign lesions, except for red blood cells, testicular germline cells, renal tubules and perineuria (26). On the other hand, its overexpression has been reported in several carcinomas such as breast, bladder, ETN and pulmonary cancers (27,28) as a result of a homeostatic disruption in normal tissue microenvironment (29). The adoption of GLUT-1 in malignant mesothelioma was first reported by Godoy et al. (28) who, investigating co-expression of GLUT isoforms in a variety of tumours, showed GLUT-1-related avidity in MPMs. In this regard transporter-encoding may have a fundamental role in cancer cell metabolism and in tumour progression as far as the maintenance of a rapid growth with invasive phenotypes (30). However, a high heterogeneity in cellular staining has been reported as avidity is stronger near necrotic areas or poorly differentiated areas (31,32), influencing its diagnostic accuracy. In our study, the pooled Se and Spe were 0.74 (95% CI: 0.57–0.85) and 0.91 (95% CI: 0.79–0.96), respectively. Moreover, significant PPV, NPV and DA were reported (0.87, 95% CI: 0.82–0.90; 0.83, 95% CI: 0.80–0.85; 0.84, 95% CI: 0.81–0.87), suggesting a role of GLUT-1 immunohistochemical assay in the diagnosis of MPMs.
GLUT-1 immunoreactivity was first reported as being nearly 100% sensitivity (19). However, subsequent studies provided conflicting results. Recently, Husain et al. (20), in a cohort study on 138 patients (78 MPM and 60 RMDs) and analysing both membrane and cytoplasmic GLUT-1 staining, reported positivity in 58% of malignant cases. Instead, Üçer et al. (24) showed a 80% GLUT-1 positivity in MPMs and a 6.6% benign lesions.
In this aspect, evidences seem to struggle with our DP rate (0.41), as a result of GLUT-1 IHC-MPM cases (n=82, 27.61%) (Figure 7). For these reasons, some Authors proposed to improve GLUT-1 accuracy by combining with other IHC markers such as IMP-3 or p-16 deletion FISH analysis. Shi et al. (33), investigating the role of the insulin-like growth factor II messenger ribonucleic acid-binding protein 3 in 109 patients (45 MPM and 64 RMDs), reported a strong cytoplasmic IMP3 staining in 73% of MPM cases, while its expression was almost undetectable in RMDs. By combining them, as reported by Lee et al. (21), FP rates significantly decreased to 4%. However, GLUT-1 potential in discriminating malignant from benign mesothelial proliferation seem to be clear, as reported by the recent BTS guidelines for the investigation and management of MPM (34).
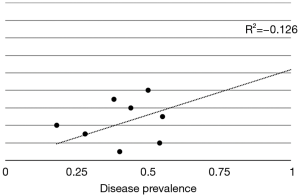
Conclusions
In conclusions, the meta-analysis seems to confirm recent finding about feasibility and accuracy of GLUT-1 immunohistochemical differentiation between MPM and benign mesothelial proliferations. However, by exploiting Forrest plots and sROC curve, properties of this transporter should be considered through the adoption of a panel of markers in order to augment diagnostic performance and thus providing pathologists high accuracy rates. Nevertheless, results reported herein may sustain a role for future general application as first-order positive assay.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.01.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tsao AS, Wistuba I, Roth JA, et al. Malignant pleural mesothelioma. J Clin Oncol 2009;27:2081-90. [Crossref] [PubMed]
- Robinson BM. Malignant pleural mesothelioma: an epidemiological perspective. Ann Cardiothorac Surg 2012;1:491-6. [PubMed]
- Geltner C, Errhalt P, Baumgartner B, et al. Management of malignant pleural mesothelioma - part 1: epidemiology, diagnosis, and staging : Consensus of the Austrian Mesothelioma Interest Group (AMIG). Wien Klin Wochenschr 2016;128:611-7. [Crossref] [PubMed]
- Alì G, Bruno R, Fontanini G. The pathological and molecular diagnosis of malignant pleural mesothelioma: a literature review. J Thorac Dis 2018;10:S276-84. [Crossref] [PubMed]
- Bolen JW, Hammar SP, McNutt MA. Reactive and neoplastic serosal tissue. A light-microscopic, ultrastructural, and immunocytochemical study. Am J Surg Pathol 1986;10:34-47. [Crossref] [PubMed]
- Churg A, Colby TV, Cagle P, et al. The separation of benign and malignant mesothelial proliferations. Am J Surg Pathol 2000;24:1183-200. [Crossref] [PubMed]
- Renshaw AA, Dean BR, Antman KH, et al. The role of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Chest 1997;111:106-9. [Crossref] [PubMed]
- Churg A, Galateau-Salle F. The separation of benign and malignant mesothelial proliferations. Arch Pathol Lab Med 2012;136:1217-26. [Crossref] [PubMed]
- Marchevsky AM. Application of immunohistochemistry to the diagnosis of malignant mesothelioma. Arch Pathol Lab Med 2008;132:397-401. [PubMed]
- Rascoe PA, Jupiter D, Cao X, et al. Molecular pathogenesis of malignant mesothelioma. Expert Rev Mol Med 2012;14:e12 [Crossref] [PubMed]
- Ikeda K, Tate G, Suzuki T, et al. Diagnostic usefulness of EMA, IMP3, and GLUT-1 for the immunocytochemical distinction of malignant cells from reactive mesothelial cells in effusion cytology using cytospin preparations. Diagn Cytopathol 2011;39:395-401. [Crossref] [PubMed]
- Attanoos RL, Griffin A, Gibbs AR. The use of immunohistochemistry in distinguishing reactive from neoplastic mesothelium. A novel use for desmin and comparative evaluation with epithelial membrane antigen, p53, platelet-derived growth factor-receptor, P-glycoprotein and Bcl-2. Histopathology 2003;43:231-8. [Crossref] [PubMed]
- King J, Thatcher N, Pickering C, et al. Sensitivity and specificity of immunohistochemical antibodies used to distinguish between benign and malignant pleural disease: a systematic review of published reports. Histopathology 2006;49:561-8. [Crossref] [PubMed]
- The Membranes of Cells - 3rd Edition [Internet]. Available online: https://www.elsevier.com/books/the-membranes-of-cells/yeagle/978-0-12-800047-2
- Kobayashi M, Kaida H, Kawahara A, et al. The relationship between GLUT-1 and vascular endothelial growth factor expression and 18F-FDG uptake in esophageal squamous cell cancer patients. Clin Nucl Med 2012;37:447-52. [Crossref] [PubMed]
- Jiwa LS, van Diest PJ, Hoefnagel LD, et al. Upregulation of Claudin-4, CAIX and GLUT-1 in distant breast cancer metastases. BMC Cancer 2014;14:864. [Crossref] [PubMed]
- Lee DW, Chong GO, Lee YH, et al. Role of SUVmax and GLUT-1 Expression in Determining Tumor Aggressiveness in Patients With Clinical Stage I Endometrioid Endometrial Cancer. Int J Gynecol Cancer 2015;25:843-9. [Crossref] [PubMed]
- Monaco SE, Shuai Y, Bansal M, et al. The diagnostic utility of p16 FISH and GLUT-1 immunohistochemical analysis in mesothelial proliferations. Am J Clin Pathol 2011;135:619-27. [Crossref] [PubMed]
- Kato Y, Tsuta K, Seki K, et al. Immunohistochemical detection of GLUT-1 can discriminate between reactive mesothelium and malignant mesothelioma. Mod Pathol 2007;20:215-20. [Crossref] [PubMed]
- Husain AN, Mirza MK, Gibbs A, et al. How useful is GLUT-1 in differentiating mesothelial hyperplasia and fibrosing pleuritis from epithelioid and sarcomatoid mesotheliomas? An international collaborative study. Lung Cancer 2014;83:324-8. [Crossref] [PubMed]
- Lee AF, Gown AM, Churg A. IMP3 and GLUT-1 immunohistochemistry for distinguishing benign from malignant mesothelial proliferations. Am J Surg Pathol 2013;37:421-6. [Crossref] [PubMed]
- Kuperman M, Florence RR, Pantanowitz L, et al. Distinguishing benign from malignant mesothelial cells in effusions by Glut-1, EMA, and Desmin expression: an evidence-based approach. Diagn Cytopathol 2013;41:131-40. [Crossref] [PubMed]
- Lagana SM, Taub RN, Borczuk AC. Utility of glucose transporter 1 in the distinction of benign and malignant thoracic and abdominal mesothelial lesions. Arch Pathol Lab Med 2012;136:804-9. [Crossref] [PubMed]
- Üçer Ö, Dagli AF, Kilicarslan A, et al. Value of glut-1 and koc markers in the differential diagnosis of reactive mesothelial hyperplasia, malignant mesothelioma and pulmonary adenocarcinoma. Turk Patoloji Derg 2013;29:94. [Crossref] [PubMed]
- Olson AL, Pessin JE. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr 1996;16:235-56. [Crossref] [PubMed]
- Younes M, Lechago LV, Somoano JR, et al. Wide expression of the human erythrocyte glucose transporter Glut1 in human cancers. Cancer Res 1996;56:1164-7. [PubMed]
- Mellanen P, Minn H, Grénman R, et al. Expression of glucose transporters in head-and-neck tumors. Int J Cancer 1994;56:622-9. [Crossref] [PubMed]
- Godoy A, Ulloa V, Rodríguez F, et al. Differential subcellular distribution of glucose transporters GLUT1-6 and GLUT9 in human cancer: ultrastructural localization of GLUT1 and GLUT5 in breast tumor tissues. J Cell Physiol 2006;207:614-27. [Crossref] [PubMed]
- Merrall NW, Plevin R, Gould GW. Growth factors, mitogens, oncogenes and the regulation of glucose transport. Cell Signal 1993;5:667-75. [Crossref] [PubMed]
- Newsholme EA, Board M. Application of metabolic-control logic to fuel utilization and its significance in tumor cells. Adv Enzyme Regul 1991;31:225-46. [Crossref] [PubMed]
- Brown RS, Leung JY, Kison PV, et al. Glucose transporters and FDG uptake in untreated primary human non-small cell lung cancer. J Nucl Med 1999;40:556-65. [PubMed]
- Mamede M, Higashi T, Kitaichi M, et al. [18F]FDG uptake and PCNA, Glut-1, and Hexokinase-II expressions in cancers and inflammatory lesions of the lung. Neoplasia 2005;7:369-79. [Crossref] [PubMed]
- Shi M, Fraire AE, Chu P, et al. Oncofetal protein IMP3, a new diagnostic biomarker to distinguish malignant mesothelioma from reactive mesothelial proliferation. Am J Surg Pathol 2011;35:878-82. [Crossref] [PubMed]
- Woolhouse I, Bishop L, Darlison L, et al. British Thoracic Society Guideline for the investigation and management of malignant pleural mesothelioma. Thorax 2018;73:i1-30. [Crossref] [PubMed]
Cite this article as: Barone M, Divisi D, Zaccagna G, Guetti L, Camplese P, Di Nuzzo D, Cipollone G, Crisci R, Mucilli F. Diagnostic performance of glucose transporter-1 immunohistochemistry in malignant pleural mesothelioma: a meta-analysis. Shanghai Chest 2019;3:21.