The place for minimally invasive surgery in extended resections for lung cancer in the management of T4 disease
For a long time, T4 disease of lung cancer was considered as a non-surgical problem, since the early trials of TNM staging system where T4 descriptor was tumor extends beyond the lung. During the late 80s, the previous T3 descriptors were split between the T3 category and the new T4 category on the basis that the former would retain those tumors that were candidates for complete resection while the latter category would contain tumors which were considered to be inoperable (1). This limited the role of surgery for those patients to the diagnostic intents in many centers except for a few publications that demonstrate the feasibility of complete resection of those advanced stages with a relatively high rate of mortality and morbidity. Pitz et al. emphasized his retrospective study on 89 patients with T4 non-small cell lung cancer (NSCLC) where they were able to perform complete resection on 38.2% of the patients. Mortality was 19.1% during the hospital stay. Among all hospital survivors, mean 5-year survival was 23.6%. However, it is worth mentioning that 5-year survival was 46.2% for patients with complete resection while it was 10.9% for patients with an incomplete resection (2). They considered a complete resection to be the main factor for an improved survival and a decrease in overall mortality.
The extent of T4 tumors remains unclear with the heterogeneous group for involvement even with the 7th edition of TNM staging for NSCLC where T4 disease carries the tumors of any size that invades any of the following: mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body, carina. Or if there is a separate tumor nodule(s) in a different ipsilateral lobe to that of the primary (3).
For various reasons, surgeons have been reluctant to apply video-assisted thoracoscopic surgery (VATS) techniques to the resection of advanced lung cancer. These include concerns about getting an oncologically sound operation, the feasibility of doing an R0 resection, issues about safety and overall long-term survival benefits in those advanced stages. As surgical techniques have become refined as well as there being improvements in terms of training and mentoring. Moreover, there have been many other aspects that have increased these benefits. For example, the introduction of high volume of cases in large centers, technological progression in high definition cameras, 3D monitors, specific instruments for thoracic minimal invasive operation other than those of laparoscopy that were used previously at the start of VATS program in most centers. As well as, vascular clips and angulated small profile staplers have made this approach much more safe, even in the much less invasive uniportal VATS approach and the new technique of subxiphoid approach.
The evidence behind minimal invasive approach for T4 disease
Early reports of VATS lobectomy have been limited to early-stage lung cancer, and thoracotomy remains the standard of care for locally advanced tumors for a decade afterward (4,5). With the development of experience more surgeons start to investigate the feasibility of VATS for performing a complete resection for a locally advanced disease. This is particularly with the add-on value that VATS demonstrates not only in decreasing postoperative pain and hospital stay. It also the improved immune response which becomes increasingly important in advanced disease of NSCLC, as most of those patients will need chemotherapy and or target therapy (6). Hennon et al. in 2011 retrospectively evaluated 125 patients with a locally advanced NSCLC of which 73 patients had a thoracoscopic resection. Median operative time, blood loss, significant complications, and duration of hospital stay were not statistically significantly different between patients who had underwent a thoracoscopic and open resection. A higher percentage of patients who got a thoracoscopic lobectomy were able to receive adjuvant therapy when compared to the open surgery group (37.2% vs. 5.2%; P=0.006). Mean operation time for VATS resections was 231 min, which is reasonable for advanced-stage resections. Although the primary goal of Hennon et al. was safety, they extend their publication to include the differences between the thoracoscopic and open surgery groups in overall survival which was 43.7 months in thoracoscopic group vs. 22.9 months in open surgery group; and disease-free survival [34.7 vs. 16.7 months; which they found to be not significant (P=0.59 and 0.84 respectively)] (7).
Villamizar et al. in 2013 tried to demonstrate the impact of T-status and N-status on the outcomes after a thoracoscopic lobectomy. Despite the title of the article, they concluded that “lung cancers that are central, clinically node positive, or larger than 3 cm does not confer to an increased morbidity when compared to peripheral, clinical N0 cancers that are smaller than 3 cm” without a clear percentage of how many cases of T3 and T4 they operate upon but they only demonstrate a percentage of 30% (7 of 23 patients) for tumors larger than 7 cm (8).
Since the emerging of uniportal VATS lobectomy by Gonzalez et al. in 2011 (9) uniportal VATS became more popular worldwide with numerous workshops and live surgeries by the inventor of this approach (10). Gonzalez-Rivas et al. in 2014 demonstrated their retrospective study on uniportal VATS for advanced lung cancer. They hypothesized that it is crucial to reduce the surgical aggressiveness as much as possible, especially in advanced stage lung cancer patients, as their immune system is weakened either by the disease itself or by chemo-radiotherapy (11). They operated on 43 patients with advanced disease out of the 130 total patients with NSCLC during the periods between 2010 and 2012. The advanced group of patients included complex cases such as lobectomies with vascular sleeve resections, bronchial sleeve resections, chest wall resection, lobectomies after concurrent chemo-radiotherapy, redo-VATS, completion pneumonectomy or sulcus tumor after neoadjuvant treatment. Conversion rate, surgical time and the median number of lymph nodes were higher in advanced group (1.1% vs. 6.5%, P=0.119, 144.9±41.3 vs. 183.2±48.9 minutes, P<0.001 and 14 vs. 16, P=0.004) respectively. Worth mentioning, 67.4% of patients advanced disease group received induction chemo or chemo-radiotherapy before surgery. The overall 30-month survival of all patients included in that study was 85%. Of which the 30-month survival was 90.4% for early stages and 73.7% for advanced cases.
In a more recent publication by the Italian VATS group, Gonfiotti et al. represented 454 patients with locally advanced disease (group B) and a total of 3,266 (87.8%) patients as early-stage NSCLC (group A). Group B was associated with a longer operative time, more loss of blood and increased conversion rate to open approach (9.3% vs. 13.0%, P=0.018). However, the mortality rate and the hospitalization were not statistically different between the two groups (P=0.880 and 0.660, respectively); complication rate was statistically higher in group B (30.4% vs. 37.0%; P=0.04), and it was not a surprise that patients of group B who required a conversion to open surgery had a statistically significantly longer operative time (P<0.01), higher blood loss (P<0.01), and hospital stay (P<0.01). However, the two groups did not significantly differ in overall morbidity (35.5% vs. 28.0%) when compared to those who completed their operation using VATS. In this study, only 8.1% of both groups were operated upon via uniportal VATS approach and authors did not mention any differences in the outcome or conversion rate between the uniportal and the multi-port VATS approach groups (12). Gonfiotti et al. presented reduced incidences of conversion to open thoracotomy, and the study had compared mortality to previous publications, and they managed to get complete resection R0 in 89.9% of the advanced group, but they failed to mention the overall survival for those patients enrolled in the study.
We think that it is unfair to compare operative time, blood loss and even hospital stay between locally advanced NSCLC and early stages as they surely will vary as the extension of resection and the bulk of the tumor itself in most cases will make the operation more lengthy, bloody, higher demand, and a longer hospital stay, while most of the surgical literature demonstrates this comparison. On the other hand, few publications were interested in demonstrating the difference in outcomes and patient experiences between chemoradiotherapy and surgery for advanced stage lung cancer. Trails comparing surgery plus chemotherapy and radiotherapy to surgery alone were terminated early (13,14)
Patient selection
Patient selection criteria for whom to apply a thoracoscopic approach to for advanced-stage lung cancer resection are not demarcated, and it is impacted greatly by the ability of the surgeon, patients’ general condition, performance status and it is impossible to be certain the degree of the invasion during the preoperative stage. the degree of invasion which is sometimes impossible to certain preoperatively.
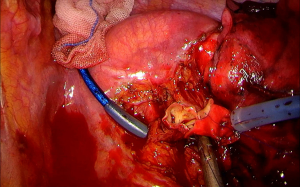
Preoperative T4 disease is not a contraindication for surgery because the patient may have a lower-stage disease intraoperative, with potential for resection; or they may have limited tumor involvement that could be resected completely (Figure 1). However, the involvement of mediastinal nodes is a strong predictor for systemic failure, even with an extended surgery, so it is important to diagnose and exclude patients with N2/N3 nodes whenever possible. In all patients who were undergoing lung cancer staging, the metastatic workup was essential, but even more so in the patients with locally advanced tumors who have a higher probability of distant disease and higher risk from surgical resection (15).
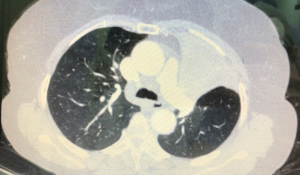
All advanced NSCLC patients eligible for surgery should get a pre-operative evaluation. Including echocardiography to evaluate cardiac function, contrast-enhanced thoracic and abdominal (CT) scan, brain CT scan, and PET-CT scan. Endobronchial ultrasound-guided transbronchial fine-needle aspiration (EBUS-TBNA) or mediastinoscopic biopsy should be performed before surgery in case of mediastinal lymph node enlargement or hyperactivity on the CT or PET-CT scan. Restaging after induction therapy is usually completed with thoracic and abdominal imaging, EBUS-FNA or mediastinoscopy.
These patients must have adequate pulmonary and cardiac functions with good performance status and minimal comorbidities. Relative contraindications include age above 70, poor functional condition and cardiac or pulmonary limitations. Additional testing may be needed preoperative for borderline patients such as cardiopulmonary exercise testing.
Surgical technique
The learning curve required for advanced thoracoscopic cases has been aided by improved video, stapling, hemostatic, and retraction technologies. Excellent exposure is enabled by high-definition camera systems that allows viewing from awkward angles. Endoscopic staplers have been modified to facilitate negotiation of small pulmonary vessels. Improved local hemostatic technologies are useful when dealing with diffuse oozing from extrapleural or inflammatory dissections after induction therapy (16).
Resection can be considered complete when (I) the surgeon is sure that all known diseases are removed; (II) resection margins are histopathologically clear; and (III) the highest mediastinal lymph node are negative by microscopic examination (2).
Several surgical techniques were employed for a minimally invasive resection for the advanced stages depending on the infiltrated part and the intended operation.
Bronchoplastic procedures
For a bronchoplastic procedure, Gonzalez-Rivas et al. recommend proper placement of the port in the cases of the uniportal approach as the port should be on the 4th intercostal space more towards the anterior axillary line (17). This placement helps to use the needle holder parallel to the hilum while doing the anastomosis (Figure 2). Using a wound protector is preferable in those cases, as fatty tissue might interfere with suture threads, as well as using a continuous absorbable suture (polydioxanone, PDS 3/0) which makes the thread movement easier (18).
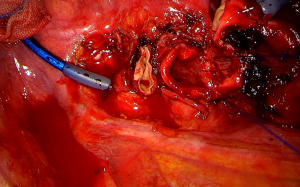
Vascular sleeve resection
Vascular reconstruction either as partial resection of the pulmonary artery or end-to-end anastomosis (Figure 3) was reported via a minimally invasive approach (17). Usually, surgeon prefers to get a proximal control or even an intrapericardial control in some cases early in operation and to make vascular reconstruction as the last step of lobectomy to gain more space for suturing after the division of vein, bronchus, and fissure (18). Vascular control can be done using a thoracoscopic vascular clamp or a bulldog clamp or the more recent use of a tourniquet to get more space especially in uniportal VATS approach (19). Double sleeve resection was also reported in few publications where surgeon need to do bronchial and vascular sleeve using the same sequence of that of the vascular sleeve (20).
Chest wall invasion
In cases of chest wall involvement (Figure 4), Bayarri et al. advocated the use of single port VATS to ensure the chest wall involvement that appears on CT scan and to delineate the exact boundaries and safety margins to avoid extensive unnecessary thoracotomies. They started the procedure by a single port VATS, accurate determination of chest wall involvement was made, a skin incision just above the area was performed. A resection of the chest wall was first made, then completed the en-bloc resection through the incision that was made just above the chest wall involvement area. The study claimed this decreased the chest wall trauma and avoided rib spreading as well (21).
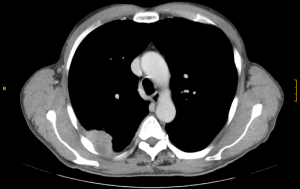
Gonzalez-Rivas et al. describes another technique that was also started with a single port VATS to delineate the lesion on chest wall from inside, then after opening a posterior incision just above the lesion to excise the chest wall segment involved then they complete the lobectomy via the uniportal incision (22).
Jaus et al. named their technique the hybrid treatment, by doing three port VATS incisions to know the exact invasion site of the chest wall using VATS guidance. Then, they proceeded with a pulmonary resection and finally did the chest wall resection. They recommended the use of needles which penetrate the chest wall from outside under the VATS guidance to mark the extent of chest wall resection (23). Another publication by Gonzalez-Rivas et al. describes the uniportal technique where they use only one port 5 cm in length. They perform the lobectomy first then use a combination of thoracoscopic and conventional rib cutter to remove the involved segment (24).
Same techniques of localizing the lesion and adjusting the incision were used in some reports of surgery for Pancoast tumor (25-27).
Conclusions
The decision to proceed with surgery for advanced lung cancer should be offered after thorough investigation and diagnostic workup. Safety considerations should dictate operation by an experienced surgeon starting, at least, with thoracoscopic assessment then conversion during the operation if needed. Minimal invasive approaches should be the first choice for a debilitated patient with advanced stage lung cancer who requires multimodal therapy or who considered to be too frail for open thoracotomy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Alper Toker and Alan D. L. Sihoe) for the series “Extended resections for lung cancer” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.04.02). The series “Extended resections for lung cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hermanek P, Scheibe O, Spiessl B, et al. TNM classification of malignant tumors: the new 1987 edition. Radiobiol Radiother (Berl) 1987;28:845-6. [PubMed]
- Pitz CC, Brutel de la Riviere A, van Swieten HA, et al. Results of surgical treatment of T4 non-small cell lung cancer. Eur J Cardiothorac Surg 2003;24:1013-8. [Crossref] [PubMed]
- Goldstraw P. The 7th Edition of TNM in Lung Cancer: what now? J Thorac Oncol 2009;4:671-3.
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [Crossref] [PubMed]
- Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol 2011;18:3732-6. [Crossref] [PubMed]
- Villamizar NR, Darrabie M, Hanna J, et al. Impact of T status and N status on perioperative outcomes after thoracoscopic lobectomy for lung cancer. J Thorac Cardiovasc Surg 2013;145:514-20; discussion 520-1. [Crossref] [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Editorial Office. Uniportal VATS live surgery around the world. Video-assist Thorac Surg 2016;1:5. [Crossref]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641-8. [PubMed]
- Gonfiotti A, Bongiolatti S, Bertolaccini L, et al. Thoracoscopic lobectomy for locally advanced-stage non-small cell lung cancer is a feasible and safe approach: analysis from multi-institutional national database. J Vis Surg 2017;3:160. [Crossref] [PubMed]
- Nagai K, Tsuchiya R, Mori T, et al. A randomized trial comparing induction chemotherapy followed by surgery with surgery alone for patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209). J Thorac Cardiovasc Surg 2003;125:254-60. [Crossref] [PubMed]
- Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673-80. [Crossref] [PubMed]
- DiPerna CA, Wood DE. Surgical management of T3 and T4 lung cancer. Clin Cancer Res 2005;11:5038s-44s. [Crossref] [PubMed]
- Hennon MW, Demmy TL. Video-assisted thoracoscopic surgery (VATS) for locally advanced lung cancer. Ann Cardiothorac Surg 2012;1:37-42. [PubMed]
- Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resections. Eur J Cardiothorac Surg 2016;49:i6-16. [PubMed]
- Gonzalez-Rivas D, Yang Y, Sekhniaidze D, et al. Uniportal video-assisted thoracoscopic bronchoplastic and carinal sleeve procedures. J Thorac Dis 2016;8:S210-22. [PubMed]
- Jiang L, Wu L, Roque SR, et al. A novel tourniquet technique for transient pulmonary artery occlusion during video-assisted thoracoscopic surgery. J Thorac Cardiovasc Surg 2018;156:816-8. [Crossref] [PubMed]
- Abu Akar F, Yang C, Lin L, et al. Intra-pericardial double sleeve uniportal video-assisted thoracoscopic surgery left upper lobectomy. J Vis Surg 2017;3:51. [Crossref] [PubMed]
- Bayarri CI, de Guevara ACL, Martin-Ucar AE. Initial single-port thoracoscopy to reduce surgical trauma during open en bloc chest wall and pulmonary resection for locally invasive cancer. Interact Cardiovasc Thorac Surg 2013;17:32-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Single-incision thoracoscopic right upper lobectomy with chest wall resection by posterior approach. Innovations (Phila) 2013;8:70-2. [PubMed]
- Jaus MO, Forcione A, Gonfiotti A, et al. Hybrid treatment of T3 chest wall lung cancer lobectomy. J Vis Surg 2018;4:32. [Crossref] [PubMed]
- Gonzalez-Rivas D, Xie B, Yang Y, et al. Uniportal video-assisted thoracoscopic lobectomy with en bloc chest wall resection. J Vis Surg 2015;1:7. [PubMed]
- Kawai N, Kawaguchi T, Yasukawa M, et al. Less Invasive Approach to Pancoast Tumor in a Partitioned Incision. Ann Thorac Cardiovasc Surg 2017;23:161-3. [Crossref] [PubMed]
- Rosso L, Nosotti M, Palleschi A, et al. VATS lobectomy combined with limited Shaw-Paulson thoracotomy for posterolateral Pancoast tumor. Tumori 2016; [Crossref] [PubMed]
- Caronia FP, Fiorelli A, Ruffini E, et al. A comparative analysis of Pancoast tumour resection performed via video-assisted thoracic surgery versus standard open approaches. Interact Cardiovasc Thorac Surg 2014;19:426-35. [Crossref] [PubMed]
Cite this article as: Elkhayat H, Gonzalez Rivas D. The place for minimally invasive surgery in extended resections for lung cancer in the management of T4 disease. Shanghai Chest 2019;3:23.