Advanced resections in minimally invasive surgery: robotic pneumonectomy
Introduction
Over the last three decades there was a worldwide diffusion of minimally invasive techniques in performing anatomical lung resections (lobectomy and segmentectomy) for early-stage lung cancer. Video-assisted thoracoscopic surgery (VATS) and more recently robotic-assisted surgery have become routine approaches in the surgical treatment of non-small cell lung cancer in stage I and II. These minimally invasive approaches have shown an increased benefit for patients because they are associated with minor morbidity, and less postoperative hospital stay compared to a conventional thoracotomy (1-4). Moreover, they have been shown to allow similar oncologic results (5) and to be superior for elderly people (6) and for patients with reduced pulmonary function (7). Although these evidences demonstrate a real benefit of minimally invasive procedures, recent studies show that the majority of lobectomies for early stage lung cancer are performed by open technique (8,9). Thus, the idea of approaching with a vats or robotic approach a patient who may require a pneumonectomy is likely to be met with skepticism, hesitation, and disapproving looks from many thoracic surgeons.
Despite the rapid expansion of robotic approach in performing lung resection with mature results and large experience to date the majority of oncological centers have reported on lobectomy (10-14) and segmentectomy (15-17), but few centers have published on robotic pneumonectomy (18-21).
In this article we describe indications for robotic pneumonectomy, the technical set up of the robot, the operating technique, and we discuss benefit, and disadvantages of this minimally invasive approach.
Indications
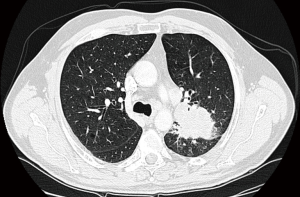
Indications for robotic pneumonectomy are the followings: (I) lung cancer or associated lymphadenopathy involving an important portion of the pulmonary artery and when vascular reconstruction is not feasible; (II) high selected patients with synchronous ipsilateral multilobar malignancies; (III) tumors less than 5–6 cm extended into different lobes across the (Figure 1).
Preoperative work-up
Preoperative evaluation is similar for all our pulmonary resections, and includes computer tomography of the chest, abdomen and brain, positron emission tomography imaging, pulmonary function tests, quantitative perfusion scan, cardiac evaluation including echocardiogram. Bronchoscopy with endobronchial ultrasound evaluation is performed to obtain cito/istological diagnosis of the tumor and to evaluate mediastinal lymph nodes.
Surgical technique
Anesthetic set up, patient positioning, and robotic set up
A double lumen endotracheal tube is positioned for obtaining a single-lung anesthesia. A central venous access and invasive blood pressure control are installed.
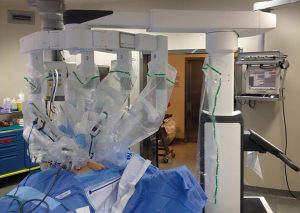
Patient position is lateral decubitus with the arm in front of the patient (Figure 2A) and the hip positioned at the same level of the chest (Figure 2B) allowing the camera to move without catching on the hip. Anesthesia is located to the face side of the patient to allow the access to the endotracheal tube without problems. If conversion to open surgery is required without urgency, the arm is moved up and a lateral muscle-sparing thoracotomy is performed. The robot cart is located at the patient’s head side, with the console in the same room (Figure 3).
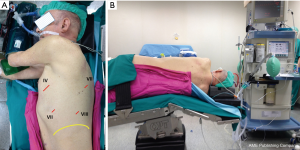
A complete four-arm approach is used to complete robotic pneumonectomy. A 4-cm minithoracotomy is usually performed in the fourth intercostal space, anteriorly (Figure 2A) without spread of the ribs with the use of a skin retractor. Through this incision the camera is introduced into the chest; pleural cavity is explored for evaluating the presence of adhesions, pleural or parenchymal nodules, the exclusion of the lung, and other possible contraindication to continue the scheduled surgical procedure. The camera port is introduced in the 7th intercostal space at the posterior axillary line, and two other 7-mm ports are placed in the 8th–9th intercostal space, and at the 7th intercostal space in the posterior aspect of the scapula’s tip, respectively (Figure 2A).
After docking the robot, the robotic instruments are inserted under vision into the pleural cavity.
Left pneumonectomy
Veins isolation and resection
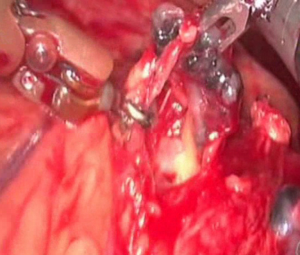
For the left pneumonectomy (see Figure 4), the camera port is positioned few centimeters posteriorly respect to the right side to avoid that the presence of the heart may render difficult the vision of the anatomical structures of the hilum. The first step is the isolation of the inferior vein. After moving the lung upward, the inferior pulmonary ligament is resected by hook cautery. At this step, the subcarinal lymph-node dissection is accomplished by opening the posterior mediastinal pleura. The inferior vein is isolated and, after being surrounded by a vessel loop, it is sectioned with En-doGia Roticulator vascular cartridge inserted by the assistant surgeon through the utility incision after the removal of the robotic arm and controlled (Figure 5A). With the use of the new Da Vinci Xi machine, this suture is performed by an EndoWrist robotic stapler directly controlled by the surgeon at the console. The superior vein is prepared in the same way: the vessel is encircled (Figure 5B) and transected with endostapler or Endowrist stapler with vascular cartridge introduced through the posterior trocar incision of the 8th intercostal space.

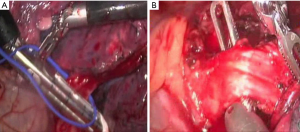
Artery isolation and resection
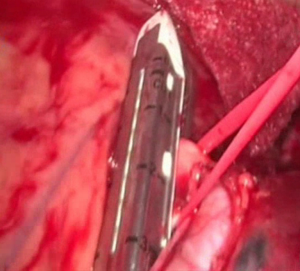
The pulmonary artery is visualized easily after the resection of the pulmonary veins. To complete di liberation of the main left pulmonary artery, the node dissection of the station #5 is performed (Figure 6) by using the hook cautery and the Cadiere forceps so that the plane between the main bronchus and the artery is performed. Once the artery has been encircled by a vessel loop, the stapling articulated device (EndoGia or EndoWrist) is introduced by the posterior trocar and the artery is transected (Figure 7). The lung retraction is maintained by Cadiere forceps introduced through the posterior robotic arm.
Bronchus isolation and resection
The last step is the bronchus isolation. Lymph nodes of station #7 have been removed before or they may be removed during this step as previously described. Lymph nodes of the tracheobronchial angle are removed following the same steps of open surgery. The bronchus is isolated and surrounded by using two Cadiere forceps (Figure 8). The stapler is introduced through the posterior port at the 8th intercostal space by using a 45 mm green cartridge.
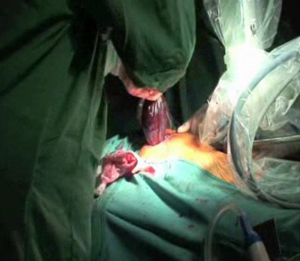
The lung is removed from the chest through the anterior mini-thoracotomy without rib spreading using an EndoCatch (Autosuture, Covidien) (Figure 9). One 32 Fr pleural drain is positioned through the camera port.
Right pneumonectomy
The different steps for performing a right pneumonectomy are similar to the left procedure. The first step is the isolation of the veins by using the hook cautery or Cadiere forceps introduced through the utility incision, and two Cadiere forceps one of which is used for retracting the lung for a better exposure. A Maryland bipolar forceps may be used at the place of the cautery hook in case of the phrenic nerve is attached to the hilar structures, in order to dissect the tissue around the superior pulmonary vein. After the dissection of the inferior pulmonary ligament, the inferior vein is isolated and sectioned with endostapler or EndoWrist introduced through the utility incision. The superior vein is then isolated and resected in the same way with the stapler introduced through the posterior port in the 8th intercostal space. The artery is freed by sharp and blunt dissection, it is encircled and resected with endostapler or EndoWrist introduced through the 8th intercostal space posterior port.
The lung is retracted with the Cadiere forceps and the right main bronchus is isolated. The endostapler (45 mm, green cartridge) or EndoWrist is introduced into the chest through the 8th intercostal space and the bronchus stapled and resected.
The dissection of paratracheal lymph nodes (#2R and #4R) and subcarinal station is performed by using the hook and a Cadiere forceps. Sometimes a Harmonic scalpel may be used in patients with large amount of mediastinal fat for reducing bleeding and lymphatic leakage.
Postoperative care
Patients are usually extubated in the operative room. A single chest tube connected to a water seal device without suction, with the tip in the midthoracic cavity (usually a 32 Fr), is used for balancing the mediastinum. The tube is removed at the 3rd or 4th postoperative day if the mediastinum is balanced, and there is no bleeding.
Comments
After reviewing the English literature, we found that a total of nine robotic pneumonectomies have been attempted and only six were completed by using robotic assistance (11,13,18-20), and three provided technical details of the procedures. We added another case since our previous reports (11,18); thus, we performed a total of 4 robotic pneumonectomies (3 left and one right) so that the total number rise 7 cases completely robotic performed. Giulianotti reported two conversions: one for “oncologic reasons”, and the other for a right sided pulmonary artery. In all the reported case/series, it appears that each of the surgeon had reached a large experience with the range of cases between 38 and 363; however, we were the only to indicate the prior case experience with lobectomy before we attempt the pneumonectomy.
From a technical point of view, the lateral position should be preferred because it allows a better exposure of the pulmonary veins and artery during dissection and resection. We think that the utility thoracotomy is useful because it allows the assistant surgeon to directly suck intraoperative fluids, blood, and steam produced during the use of coagulation; it allows to bring in and take out the chest cavity the resected lymph nodes and the small gauzes used during the anatomical dissection, and, above all, it allows to control directly from outside the chest an eventual vascular injury and bleeding.
In our initial experience in robotic pneumonectomy, we resected pulmonary veins as the initial step to achieve a better exposure of the pulmonary artery, but this produced a congestion of the whole lung increasing difficulties in lung mobilization. In the other 3 cases, we used a different order of vessel resections: we resected as first step the superior pulmonary vein, then the main pulmonary artery, and finally the inferior pulmonary vein. In this way, we had a decreasing of the technical inconveniences and the operative time.
In our experience of 4 cases of robotic pneumonectomy, the mean operative time was 225 minutes (range, 170–320 minutes). All patients were extubated immediately after the surgical operation in the operative room, and no patient was recovered at intensive care unit. In our short series, the mean postoperative stay was 7 days (range, 7–8 days). The mean number of resected lymph nodes was 25 (age, 19–34). No postoperative complications occurred in the four patients.
With the advent of new robotic instruments (Hem-o-lok for vessel closure) and robotic staplers (EndoWrist) (23), we think that the time of the operation is certainly reduced due to the great and easy maneuverability of the instruments directly controlled by the surgeon at the console.
Management of the main bronchus, particularly in the left side, pose a challenge during robotic pneumonectomy. In fact, it is important to get a proximal bronchial transection ideally behind the aortic arch, which is relatively difficult to achieve. To obtain a short bronchial stump, we applied a considerable traction on the lung to overcome the aortic arch and we used roticulator staplers (45 mm) to try and get as far down as we could.
A problem during robotic pneumonectomy is the possibility of extracting the whole lung from the thorax through a 4-cm utility thoracotomy that seems like it should be a challenge. In our experience, the extraction of the whole lung was possible in all cases by using EndoCatch, without rib spreader, and with a rocking and rotating motion.
The completeness of resection obtained by the robotic approach is equivalent to that of open surgery, including the completeness of lymph node dissection of stations #5, #6, #7, #8, and #9 on the left side, and of #2, #4, #7, #8, and #9 on the right side.
In conclusion, although large experience has been gained with robotic lug resection, robotic pneumonectomy remains an operation that is still undergoing development and should be reserved for centers with high experience. Selection of patients who underwent robotic pneumonectomy is crucial, and surgeons who performed this challenging procedure should have a lot of experience in robotic surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Shanghai Chest for the series “Minimally Invasive Thoracic Oncological Surgery”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.04.01). The series “Minimally Invasive Thoracic Oncological Surgery” was commissioned by the editorial office without any funding or sponsorship. DG served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Shanghai Chest from Jul 2017 to Jun 2019. LS served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Shanghai Chest from Jul 2017 to Jun 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8.
- Cao C, Manganas C, Ang SC, et al. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann Cardiothorac Surg 2012;1:3-10. [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-assisted, video-assisted thoracoscopic and open lobectomy: propensity-matched analysis of recent premier data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and not randomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Port JL, Mirza FM, Lee PC, et al. Lobectomy in octogenarians with non small cell lung cancer: ramifications of increasing life expectancy and the benefits of minimally invasive surgery. Ann Thorac Surg 2011;92:1951-7. [Crossref] [PubMed]
- Nakata M, Saeki H, Yokoyama N, et al. Pulmonary function after lobectomy: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2000;70:938-41. [Crossref] [PubMed]
- Gopaldas RR, Bakaeen FG, Dao TK, et al. Video-assisted thoracoscopic versus open thoracotomy lobectomy in a cohort of 13,619 patients. Ann Thorac Surg 2010;89:1563-70. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph-node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Casiraghi M, Galetta D, Borri A, et al. Ten Years' Experience in Robotic-Assisted Thoracic Surgery for Early Stage Lung Cancer. Thorac Cardiovasc Surg 2018; [Epub ahead of print]. [PubMed]
- Wei B, Eldaif SM, Cerfolio R. Robotic lung resection for non-small cell lung cancer. Surg Oncol Clin N Am 2016;25:515-31. [Crossref] [PubMed]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Nguyen D, Gharagozloo F, Tempesta B, et al. Long-term results of robotic anatomical segmentectomy for early-stage l non-small-cell lung cancer. Eur J Cardiothorac Surg 2018; [Epub ahead of print].
- Wei B, Cerfolio R. Technique of robotic segmentectomy. J Vis Surg 2017;3:140. [Crossref] [PubMed]
- Cerfolio RJ, Watson C, Minnich DJ. One hundred planned robotic segmentectomies: early results, technical details, and preferred port placement. Ann Thorac Surg 2016;101:1089-95. [Crossref] [PubMed]
- Spaggiari L, Galetta D. Pneumonectomy for lung cancer: a further step in minimally inva-sive surgery. Ann Thorac Surg 2011;91:e45-7. [Crossref] [PubMed]
- Rodriguez JR. Total port robotic pneumonectomy. Gen Thorac Cardiovasc Surg 2013;61:538-41. [Crossref] [PubMed]
- Giulianotti PC, Buchs NC, Caravagios G, et al. Robot-Assisted lung resection: outcomes and technical details. Interact Cardiovasc Thorac Surg 2010;11:388-92. [Crossref] [PubMed]
- Khan N, Fikfak V, Chan E, et al. “Five on a dice” port placement allows for successful robot-assisted left pneumonectomy. Thorac Cardiovasc Surg Rep 2017;6:e42-4. [Crossref] [PubMed]
- Galetta D, Casiraghi M, Spaggiari L. A case of left robotic pneumonectomy performed for an interlobar non-small cell lung cancer infiltrating both the left pulmonary lobes. Asvide 2019;6:119. Available online: http://www.asvide.com/article/view/31234
- Galetta D, Casiraghi M, Pardolesi A, et al. New stapling devices in robotic surgery. J Vis Surg 2017;3:45. [Crossref] [PubMed]
Cite this article as: Galetta D, Casiraghi M, Spaggiari L. Advanced resections in minimally invasive surgery: robotic pneumonectomy. Shanghai Chest 2019;3:25.