Salvage tracheal reconstruction after failed endoscopic or surgical intervention
Introduction
Airway surgery commonly concerns resection and reconstruction of benign or malignant abnormalities limited in length and complexity. Standard operative techniques permit reliably predictable outcomes after reconstruction for postintubation stenosis (1) and resection of thyroid carcinoma (2) or primary airway tumors (3). When the first intervention fails in achieving a permanently stable tracheal or bronchial airway, the patient must live with a tracheal tube or a silicone stent, and any additional complications related to airway fistula may persist. A second surgical intervention, if possible, usually recapitulates standard steps of surgical reconstruction that failed during the first operation either because of unrecognized respiratory disorders, inadequate airway mobilization or unsuitable anastomotic technique. Since second interventions pose additional risks of complication, the surgeon must possess a thorough understanding of likely causes of the original failure. Only when the conditions underlying an unfavorable outcome are resolved or improved may a different result be expected from reoperation.
We have in the past reviewed the complex management of these patients (4). In certain selected cases, second surgical reconstruction may achieve the goal of the first operation, albeit with a lower rate of success or a less favorable result (5). The purpose of this review is a detailed description of the corrective surgical steps permitting ultimate success after prior failure. Four individual patients of the senior author (HA Gaissert) at Massachusetts General Hospital (MGH) are examined with emphasis on visual records of preoperative and postoperative endoscopic findings. Each of these patients presents a facet in the management of postintubation injury, tracheoesophageal fistula and airway tumor, and all have in common young age and variable, but ultimately sufficient lengths of normal trachea. Strict criteria must be applied before any secondary intervention as successful reoperations remain a rare occurrence, mainly because of unmodifiable risk factors or insufficient airway length after failed airway reconstruction in other patients.
Methods
The study regarding case 1 and 2 was approved by the institutional review board (2009-P-000111, expires 11-8-19). Permission to publish a voice file prior to publication was received by patient 4.
Case descriptions
Patient 1: persistent tracheal postintubation stenosis after attempted surgical reconstruction
The 34 years old woman presented first at age 22 with postintubation tracheal stenosis 1 year following a motor vehicle accident with closed head trauma and mechanical ventilation. A prior tracheal resection had failed immediately requiring tracheostomy. At MGH, she underwent placement of a tracheal T-tube within months of that first failed reconstruction. Tracheal reoperation was deferred at the patient’s request due to insurance and social concerns. Eleven years later, the patient returned as a mother of three children requesting surgical reconstruction.
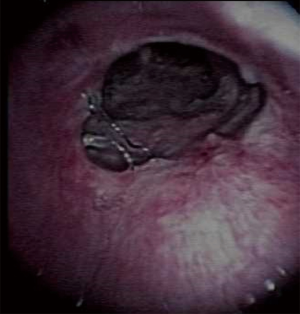
Figure 1 shows the trachea with tracheal T-tube, while Figure 2 demonstrates the airway after tube removal. Bronchoscopic measurements established 3 cm of normal airway between vocal cords and upper end of the tracheal stoma, and 4 cm of normal airway between tracheal carina and lower end of the 3.5 cm long tracheal stricture. Thus 7 cm of normal laryngotracheal airway remained in a thin, young female patient with good neck extension.


The operation was conducted via cervical collar incision. The entire airway was mobilized, freeing up the anterior tracheal surface to the carina and along the anterior aspects of both main bronchi while preserving the lateral blood supply. A great amount of scarring was encountered in the region of the anastomosis and the stoma while there were no anterior adhesions below the anastomosis, indicating an omission to mobilize these planes during the earlier procedure. The airway was divided through the lower end of the stricture, lateral traction sutures were placed, and the stricture with tracheal stoma was resected. The anastomotic sutures were placed and tied with the neck in flexion. The patient made an uneventful recovery and has returned to her usual activities. Figure 3 shows the bronchoscopic aspect of the anastomosis after 7 days and Figure 4 after 6 weeks.


Reasons for failure of first intervention: the obvious lack of airway mobilization deprived the original reconstruction of important tracheal length. Inadequate management of tension appears to be the primary cause of failure.
Comment on reoperation: reducing tension was expected as the main determinant of success, and preparations were made to add release maneuvers. However, neither suprahyoid nor pericardial release maneuvers proved necessary during the operation. Reoperations after unsuccessful prior repair have inferior outcome. In our institution, a good result is achieved in only 75% of cases after prior resection and reconstruction, with a failure rate of 5.6% and a mortality of 3.8%. In contrast, first resections had a good outcome in 86%, with a failure rate of 3.6% and a mortality of 2.1% (1).
Patient 2: Postintubation tracheal stenosis complicated by tracheoesophageal fistula
A 45-year-old man sustained a brain injury at age 9 leading to tracheostomy for postintubation tracheal stenosis. The tracheostomy tube was later removed, and a Microvasive Ultraflex tracheal stent was placed at age 35 years for obstructive symptoms. The patient developed a cough when drinking liquids 4 months prior to presentation, 10 years after placement of the stent, and wires were observed in the esophageal lumen. A 16 mm Dumon stent was placed in the trachea that subsequently occluded, leading to endotracheal intubation and aerial evacuation to MGH.
Chest radiography showed no pulmonary infiltrate. Bronchoscopic evaluation (Figure 5) demonstrated thick purulence in the right bronchial tree originating from the lower lobe. There were 7 cm of normal airway from vocal cords to upper end of a tight stricture. The length of the stricture consisted of 1 cm with stoma and a 3 mm tracheoesophageal fistula and another 2 cm of narrowed trachea. The metal stent covered a 4 cm distance from above the stricture to 1 cm above the carina, below which the trachea was normal. The patient was weaned from mechanical ventilation and prepared for surgical resection.

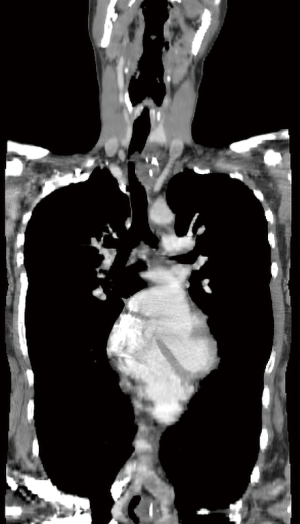
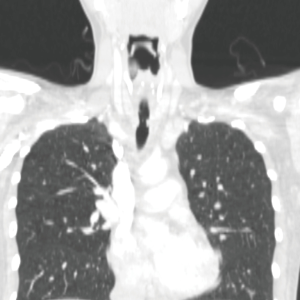
Using a cervico-mediastinal approach, the trachea was mobilized and transected below the fistula, dividing wall and metal stent. Stricture, stoma and the embedded stent were resected. The esophageal lumen was closed in two layers, and a strap muscle flap was placed over the repair (11). In judging against resecting additional trachea, the anastomosis connected to stent-bearing lower airway and sutures were passed around metal wire. The patient was immediately extubated. Respiratory secretions were managed using a mini-trach (12). He was discharged after 18 days. Figure 6 shows the bronchoscopic aspect of the anastomosis after 7 days and Figure 7 after 1 year. Figure 8 shows a coronal computed tomogram 4 years after operation. The airway remains patent, and the patient eats a regular diet.

Reasons for failure of first intervention: self-expanding metal stents are unsuitable in benign airway strictures, as confirmed by an advisory of the US Federal Drug Administration (14). Stent erosion into adjacent vascular and intestinal structures is common when left in place for longer than 2 or 3 months. Often additional normal trachea is injured by metal stents, and surgical options become limited or disappear as a result.
Comment on reoperation: in a 2003 report from our institution of 15 patients managed elsewhere with self-expanding metal stents between 1997 and 2002, 10 strictures were judged to be resectable before stent placement, while only 5 patients eventually underwent tracheal reconstruction with failure in 2 of the 5 (15). The embedded stent leaves the surgeon with few options; excision of the entire stent length raises tension at the anastomosis markedly while using stent-bearing trachea creates uncertainty about the long-term condition of the tracheal wall. Alternative palliation of a benign tracheoesophageal fistula with yet another stent is entirely unsatisfactory and may lead, as in this patient, to complications. Conceiving of such a stenting procedure as a “bridge to resection” would be misleading and harmful to patients.
Patient 3: postoperative separation of the anastomosis after resection of a long adenoid cystic carcinoma of the trachea
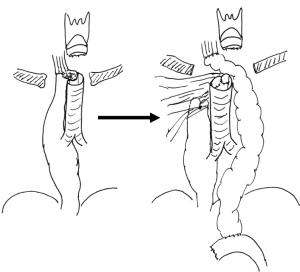
A 25-year-old woman was referred to the senior author with a 3-year history of shortness of breath and audible breathing. Computed tomography showed a long tracheal tumor (Figure 9). A bronchoscopy demonstrated a 6.5 cm long adenoid cystic carcinoma that was resected via a cervicomediastinal approach. The remaining tracheal length was measured as 5 cm. In the absence of undue tension, the anastomosis was completed at the second tracheal ring without having performed release maneuvers. One month after the original resection, the patient presented with a narrow stricture at the anastomosis (Figure 10). A tracheal T-tube was inserted to stent the anastomosis. Five months later, a re-resection was performed through a cervical collar incision without tracheal release maneuvers, and the patient recovered without event. She underwent mediastinal radiation after recovery and passed away 8 years after resection from distant metastasis. This patient was discussed in a prior publication (4).
Reasons for failure of first intervention: the assessment of tension at the anastomosis relies on surgical judgment. Despite a long tumor, the patient seemed to have a favorable anatomy: a young, thin female patient with long neck. These factors may have led the surgeon to underestimate tension and omit pericardial release maneuvers that may have avoided anastomotic separation.
Comment on reoperation: no tracheal length was sacrificed during the placement of the tracheal T-tube, and sufficient time had passed to lower anastomotic tension following a short segment tracheal re-resection.
Patient 4: complex post-esophagectomy tracheoesophageal fistula and bilateral division of recurrent laryngeal nerves
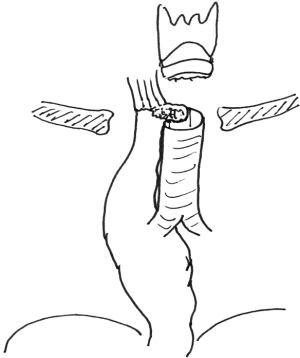
A 22-year-old woman presented to an outside hospital with intermittent dysphagia to solid food and episodic esophageal obstruction requiring endoscopic removal of food particles. The surgical reconstruction and early outcome of this case was previously published (16). Esophagoscopy demonstrated an extramucosal tumor in the muscular wall 3 cm below the cricoid. An incisional biopsy determined a granular cell tumor that was interpreted as aggressive. She underwent a transhiatal esophagectomy and, to improve the surgical margin, a window resection of the adjacent tracheal membranous portion with cartilage-to-cartilage closure (Figure 11). A primary “malignant” granular cell tumor measuring 6×3.2×3 cm and involving the periesophageal soft tissues was resected. Within 1 week, a fistula developed between the adjacent suture lines. A sequence of four surgical attempts over 5 months was made to close the fistula using local and forearm skin flaps, without success. The trachea was finally divided below the cricoid, and a distal stoma containing the fistula created. As a result, the patient was aphonic, unable to swallow and persistently threatened by aspiration.
Evaluation after transfer to MGH demonstrated an anterior tracheal and a posterior gastric lumen visible in the stoma as shown in Figure 12. On esophagoscopy (Figure 13), a gastric conduit filled with secretions was seen to disgorge fluid into the trachea; these gastric secretions rapidly reaccumulated. Swallowing was observed in the esophagus by retroflexed endoscopy through the stoma. Review of the outside pathology at MGH showed a granular cell tumor present at resection margins; absent mitosis or necrosis, the tumor was judged not to be malignant.


A staged approach was considered for surgical reconstruction. The gastric conduit was closed and separated from the trachea with interposed right pectoral muscle (Figure 14), but left in situ to support the blood supply of the trachea. During the same operation, a substernal colon conduit was connected to the cervical esophagus and drained with a Roux-en-Y jejunostomy. Her swallowing was restored, without aspiration into the blind laryngeal pouch (Figure 15).

During office laryngoscopy, glottic closure was observed during coughing despite bilateral recurrent laryngeal division, indicating the patient’s potential to protect her airway (Figure 16). In a second operation via collar incision 6 months later, the trachea was re-anastomosed to the cricoid without certainty regarding its outcome. Following delayed extubation for airway protection, the patient made an uneventful recovery. She has retained a normal voice, supported we assume by reinnervation from both superior laryngeal nerves (Figure 17). Fourteen years after reconstruction, she tolerates a regular diet. Late computed tomograms show scattered tree-in-bud infiltrates without tumor recurrence. The patient has not had pneumonia but remains at risk for deterioration of laryngeal function.


Reasons for failure of first intervention: the pathologic interpretation overestimated the malignant potential of the tumor and introduced an attitude of radical surgical removal. Unprotected suture lines and tension on the tracheal closure facilitated the formation of a fistula.
Comment on reoperation: the staging of reconstruction prioritized the control of aspiration and respiratory sepsis. Once intestinal continuity was re-established and the airway was protected, the risks bilateral vocal cord palsy presented to tracheal continuity was considered with the patient. Individual circumstance not controlled by the surgeon, mainly young patient age and compensatory re-innervation, permitted the success of tracheal reconstruction. However, this operation could well have failed due to aspiration.
Discussion
The patients discussed above represent a selected minority of patients evaluated following failed surgical reconstruction of the trachea. These patients share young age and a satisfactory residual tracheal length. The indication for reconstruction was entirely elective in two patients and non-elective, non-urgent in the other two. In none of the patients was initial failure pre-ordained due to airway disease or co-morbidity. Because of their young age, a greater risk of technical failure during the second reconstructive attempt seemed acceptable to patient and surgeon. These elevated risks were carefully explained to each patient, and the residual option in the event of a second failure consisted of placing an airway tube. Thus, the potential alternative represented an inferior quality of life compared to the anticipated favorable outcome, but not when compared to the preoperative state. In two patients who were threatened by respiratory sepsis, a second intervention was considered unavoidable.
Awareness of the precise cause of prior failure occurred in Patient 1 during reoperation, but was apparent in the others before. Essential to this assessment is the bronchoscopic examination that was available in each patient before a decision for reoperation was made. In Patients 1 and 3, the mechanism of anastomotic tension defeating the first attempt was considered limited during the second because of satisfactory residual length and a short stricture. The selection of appropriate treatment remains an individual choice in every patient with this uncommon problem. The outcome of tracheal reconstruction in patient 4 was entirely unpredictable; glottic airway obstruction and aspiration threatened the early outcome and remain a potential threat during her life span.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.07.10). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study regarding case 1 and 2 was approved by the institutional review board (2009-P-000111, expires 11-8-19). Patient 4 gave permission to publish voice and video files prior to publication. Patient 3 expired prior to publication; only non-identifiable information is shown.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grillo HC, Donahue DM, Mathisen DJ, et al. Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg 1995;109:486-92. [Crossref] [PubMed]
- Gaissert HA, Honings J, Grillo HC, et al. Segmental laryngotracheal and tracheal resection for invasive thyroid carcinoma. Ann Thorac Surg 2007;83:1952-9. [Crossref] [PubMed]
- Grillo HC, Mathisen DJ. Primary tracheal tumors: treatment and results. Ann Thorac Surg 1990;49:69-77. [Crossref] [PubMed]
- Madariaga ML, Gaissert HA. Reresection for recurrent stenosis after primary tracheal repair. J Thorac Dis 2016;8:S153-9. [PubMed]
- Donahue DM, Grillo HC, Wain JC, et al. Reoperative tracheal resection and reconstruction for unsuccessful repair of postintubation stenosis. J Thorac Cardiovasc Surg 1997;114:934-8. [Crossref] [PubMed]
- Gaissert HA, Vanni C, Madariaga ML. Trachea with tracheal T-tube in Patient 1. Asvide 2019;6:227. Available online: http://www.asvide.com/watch/32912
- Gaissert HA, Vanni C, Madariaga ML. Trachea in Patient 1 after removal of T-tube stent; visible are the stoma and the narrowed tracheal lumen below the stent. Asvide 2019;6:228. Available online: http://www.asvide.com/watch/32913
- Gaissert HA, Vanni C, Madariaga ML. Coronal computed tomogram demonstrating total tracheal length four years after second tracheal reconstruction in Patient 2. Asvide 2019;6:229. Available online: http://www.asvide.com/watch/32914
- Gaissert HA, Vanni C, Madariaga ML. Anastomotic view 6 weeks after operation in Patient 1. Asvide 2019;6:230. Available online: http://www.asvide.com/watch/32915
- Gaissert HA, Vanni C, Madariaga ML. View of trachea-esophageal fistula, embedded metal stent and luminal purulence in Patient 2. Asvide 2019;6:231. Available online: http://www.asvide.com/watch/32916
- Mathisen DJ, Grillo HC, Wain JC, et al. Management of acquired nonmalignant tracheoesophageal fistula. Ann Thorac Surg 1991;52:759-65. [Crossref] [PubMed]
- Matthews HR, Hopkinson RB. Treatment of sputum retention by minitracheotomy. Br J Surg 1984;71:147-50. [Crossref] [PubMed]
- Gaissert HA, Vanni C, Madariaga ML. Anastomotic assessment 1 week after reconstruction in Patient 2. Asvide 2019;6:232. Available online: http://www.asvide.com/watch/32917
- FDA public health notification: complications from metallic tracheal stents in patients with benign airway disorders. 2005, viewed on 29 May, 2019. Available online: http://www.jsre.org/info/0801_fda.pdf
- Gaissert HA, Grillo HC, Wright CD, et al. Complication of benign tracheobronchial strictures by self-expanding metal stents. J Thorac Cardiovasc Surg 2003;126:744-7. [Crossref] [PubMed]
- Merritt R, Zeitels SM, Austen WG Jr, et al. Staged closure of tracheogastrocutaneous fistula after esophagectomy for infiltrative granular cell tumor. J Thorac Cardiovasc Surg 2007;134:805-7. [Crossref] [PubMed]
- Gaissert HA, Vanni C, Madariaga ML. Endoscopic outpatient evaluation of cutaneous tracheal stoma and fistula in Patient 4. Asvide 2019;6:233. Available online: http://www.asvide.com/watch/32918
- Gaissert HA, Vanni C, Madariaga ML. Endoscopic view from esophagus into the fluid-filled gastric lumen in Patient 4. Asvide 2019;6:234. Available online: http://www.asvide.com/watch/32919
- Gaissert HA, Vanni C, Madariaga ML. Barium swallow sequence after colon interposition as a first stage in Patient 4. Asvide 2019;6:235. Available online: http://www.asvide.com/watch/32920
- Gaissert HA, Vanni C, Madariaga ML. Office laryngoscopy after first stage in Patient 4, demonstrating glottis closure despite bilateral recurrent laryngeal nerve division. Asvide 2019;6:236. Available online: http://www.asvide.com/watch/32921
- Gaissert HA, Vanni C, Madariaga ML. Voice recording of Patient 4, 13 years after bilateral recurrent laryngeal nerve division and two-stage reconstruction. Asvide 2019;6:237. Available online: http://www.asvide.com/watch/32922
Cite this article as: Gaissert HA, Vanni C, Madariaga MLL. Salvage tracheal reconstruction after failed endoscopic or surgical intervention. Shanghai Chest 2019;3:48.