Veno-venous extracorporeal membrane oxygenation tracheal sleeve pneumonectomy
Introduction
Tracheal sleeve pneumonectomy (TSP) is a complex surgical procedure consisting in removal of the whole lung together with the tracheo-bronchial bifurcation that is defined as the main carina. It is indicated in case of primary lung tumours of the main bronchus involving the main carina or the distal trachea or—more rarely—of primary tumours of the main carina or the distal trachea extending into one of the main bronchi (1) (Figure 1).
It can be performed—more frequently—on the right side while, on the contrary, it is quite rare on the left side. Resection of the main carina can be performed even without lung resection and—in this case—it is defined as carinal resection; this procedure, however, is very infrequent and is indicated in case of small and localized primary tumours of the carina or the distal tract of the trachea (2).
TSP represents one of the most challenging procedures for thoracic surgeons: in particular, it is very demanding—for surgeons and anaesthesiologists—the tracheo-bronchial reconstruction while providing adequate ventilation on the remaining lung after distal trachea transection.
For this purpose several techniques have been developed during years: one of the first and most used is the so called “cross-field ventilation” (CFV)—consisting in selective intubation and ventilation of the main bronchus of the remaining lung from the surgical field directly by the surgeon, with or without the help of jet ventilation; although effective and used by many surgeons, CFV makes the anastomosis between the distal trachea and the remaining main bronchus more difficult to be performed because of the presence of the tube inside the bronchus and the constant need to ventilate the lung (3).
An interesting alternative option to CFV during TSP is the use of extracorporeal membrane oxygenation (ECMO): this is a form of artificial circulatory and respiratory support based on the concept of extracorporeal circulation by using non-occlusive centrifugal pump and oxygenator which is responsible for enrichment of O2 and elimination of CO2 (4).
The aim of this paper is to analyse the use of ECMO during TSP, focusing on its indications and contraindications as well as advantages and disadvantages compared to different techniques utilized during this operation for providing adequate tissues oxygenation.
TSP
Indications and contraindications
Indications to perform a TSP are changed over the time: although formal indications are still represented by non-small cell lung cancers or other airways tumors involving the main carina, benign or inflammatory strictures (5), to date neoplastic disease represents the main indication (6).
Preoperative oncologic assessment should be performed considering that—in the vast majority of cases—we are staging a locally advanced neoplastic disease; for this reason, whole body computed tomography (CT), FDG positron emission tomography (PET) and flexible or rigid bronchoscopy are pivotal steps.
Airway endoscopy is crucial, allowing to correctly identify the degree and extension of carinal infiltration and histologically confirm endoscopic view by target biopsies—to confirm the presence of cancer—and random biopsies, 1 or 2 centimetres above and below the visible tumour, to confirm the feasibility of the procedure and a tension-free anastomosis (7).
Invasive mediastinal nodal staging by mediastinoscopy or endobronchial ultra sound (EBUS) guided transbronchial needle biopsy (TBNA) is important to rule out an N2 disease or to consider induction chemotherapy and further restaging in this subgroup of patients (8).
Preoperative functional assessment is the same of that utilized for standard pneumonectomy: complete pulmonary function tests, arterial blood gas analysis, quantitative perfusion scan and cardiopulmonary exercise test allow to correctly identify patients potentially eligible to pneumonectomy with an acceptable postoperative morbidity and mortality rate (9).
Principles of surgical technique
Several different surgical approaches to the main carina have been described, varying according to the surgeon’s preference, the neoplasm’s topography and the type of resection and reconstruction planned.
Right thoracotomy (postero-lateral or lateral total muscle sparing) is one of the most used approach to the main carina: it is ideal in case of lesions of the carina and for very extended tumours of the lower trachea (10).
Left thoracotomy is indicated in case of a tumour infiltrating the main carina and the left main bronchus when planned tracheal resection is very limited (10). Left thoracotomy with retro-aortic dissection has been also described but it does not offer good exposure for complex distal tracheal procedures (11).
Median sternotomy allows an excellent exposure of the whole trachea: the anterior aspect of the pericardium is incised between the aorta and the superior vena cava and the posterior pericardium is similarly opened; after adequate mobilization of the above-mentioned great vessels and the right main branch of the pulmonary artery, the entire trachea and the main carina are very well exposed (12).
Both clamshell and left hemi-clamshell incisions have been described for treating very extended lesions, in particular those infiltrating the left main bronchus, the main carina and a long tract of the distal trachea (10,11).
More recently, a minimally invasive video assisted approach in a non-intubated patient has been described (13) although its safety appears highly questionable (14).
Right TSP
This operation can be safely performed when no more than 4 cm of the distal trachea need to be resected. Right thoracotomy is the preferred surgical approach: the distal trachea is circumferentially isolated by careful dissection to prevent left laryngeal nerve injury. Transection of the left main bronchus is usually the first pivotal step of the procedure followed by the division of the distal tract of the trachea. At this moment—if the procedure is performed without ECMO support—left lung ventilation is provided by cross-field intubation of the left main bronchus. Subsequently, the healthy left main bronchus and the distal trachea are re-connected by an end-to-end anastomosis performed with the ventilation tube inside the left main bronchus; this step can be quite complex and time-consuming because of the restricted surgical field and the disturbing presence of the tube inside the lumen of the bronchus. Reinforcing additional single stitches are recommended to have a tension-free anastomosis as well as a pericardial fat flap interposing between vascular and bronchial anastomoses thus preventing lethal tracheo-broncho-vascular fistula (1,2).
Left TSP
This operation is performed via left thoracotomy when no more than 1 cm of trachea and of right main bronchus need to be resected below and above the edges of the left main bronchus.
Isolation is performed beneath the aortic arch, sparing the left laryngeal nerve; the Botallo’s ligament is transected to maximize exposure; the aortic arch is isolated and gradually retracted; the distal tract of the trachea is isolated after cervical flexion to reduce tracheal tension; after carinal dissection is completed, right main bronchus is then freed and clearly isolated; at the end, when resection and reconstruction appears to be feasible and safe, pulmonary artery and veins are divided.
Stay sutures are placed in the left lateral aspect of the trachea and in the median wall of the right main bronchus; subsequently, the right main bronchus is divided and—if no ECMO support is programmed—CFV is started through the right main bronchus; the distal part of the trachea is divided and tracheobronchial anastomosis is performed by using tapes around the aorta for alternate retraction of the aortic arch.
Median sternotomy is required to perform left TSP when a greater amount of the distal trachea is infiltrated. Isolation and mobilization of trachea is started cranially and then caudally extended. Isolation of right main bronchus is then completed. As previously described, the Botallo’s ligament is transected for a better exposure, after opening the pericardium. The dissection of the right main bronchus is extended up to the origin of the right upper lobe bronchus. Similarly, pulmonary vessels division is performed only after resection and reconstruction appears to be feasible and safe. Trachea and right main bronchus are circumferentially transected and the anastomosis between the distal tracheal and the healthy left main bronchus is performed thanks to standard CFV, if no ECMO is provided (1,2).
ECMO
ECMO is an artificial circulatory and respiratory support system whose use is indicated in case of patients suffering from respiratory or cardiac failure; ECMO can be used as a temporary bridge until restoration of cardiopulmonary function or—in case of untreatable respiratory failure—to lung transplantation (15). The present use of ECMO in thoracic—non cardiac—surgery is limited to post-operative acute respiratory distress syndrome (ARDS), in particular in single-lung patient following pneumonectomy, lung transplantation, and congenital pathologies of the airways in neonates (16). In the present article we focus on the intraoperative use of ECMO for adult TSP for cancer.
Basic principles
ECMO relies on the concept of extracorporeal circulation by using non-occlusive centrifugal pump and oxygenator: in this way ECMO allows blood enrichment of O2 and elimination of CO2. Depending on the type and site of vascular cannulation, ECMO can be defined as central or peripheral as well as veno-venous (VV) or veno-arterial (VA) (Figure 2); moreover, it can provide partial or total circulatory support (17). VA ECMO can support either the cardiac and respiratory functions by providing gas exchange and cardiac hemodynamic support; VA ECMO is indicated when both respiratory and cardiac support is needed. VV ECMO supports exclusively the pulmonary function by providing blood oxygenation and CO2 removal.
ECMO-assisted TSP
The first resection of the main carina and main stem bronchi—for a recurrent cylindroma—with the use extra circulatory support was performed on December 21, 1959 and reported by Woods et al. in 1961 (18). Since then, few cases of airway resections have been performed with the use of cardiopulmonary bypass (CPB) or ECMO and these procedures, due to the complexity and rarity of cases, are nowadays performed only in few, high specialized centres.
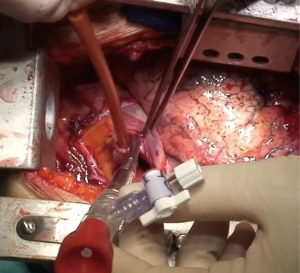
VV ECMO supports exclusively the respiratory function by oxygenation and CO2 extraction; it is therefore indicated only in patients without cardiac instability or failure. Venous blood is aspired to the ECMO reservoir and—after passing through the oxygenator—it is re-inserted into the venous system by a pump. Usually, femoro-jugular of jugulo-femoral cannulation is performed, although double lumen single cannulas have been developed for exclusive jugular vein cannulation (Figures 3,4).


The absence of arterial cannulation reduces significantly vascular local complications that can be observed during or after VA ECMO or standard CPB.
There are several advantages of ECMO versus standard CPB during carinal pneumonectomy: thanks to the recent use of ECMO heparin-coated cannulas, ECMO requires low anticoagulation with anti-coagulation time (ACT) usually ranging between 160 and 200 and low dose of heparin (less than 1,000 IU) at cannulation time; moreover, the risk of ECMO-related thrombo-embolic complications is relatively low, due to the short time needed to perform tracheo-carinal reconstruction.
The ECMO support, in fact, is only needed to safely perform the airway anastomosis and it is limited to this part of the procedure, usually lasting less than 30 minutes. Although a theoretical risk of tumour cell scattering has been reported in the past (21) there is no evidence about higher risk of neoplastic cell seeding during procedures requiring cardio circulatory support like ECMO or CPB; moreover, starting the ECMO support only after pulmonary vessel ligation and lung extraction, further reduce the supposed risk of cell scattering; we are now investigating this aspect in a prospective, double arm, observational study comparing patients receiving intraoperative cardiorespiratory support with patients—with similar oncologic disease and extension—operated without the need of ECMO or CPB (22).
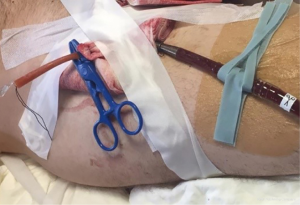
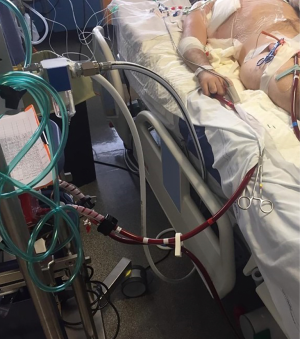
One of the most important advantage of ECMO in the setting of carinal pneumonectomy is the absence of disturbing tubes and lines, in the operative field, when cannulas are inserted peripherally; moreover, peripheral ECMO can be post-operatively prolonged during intensive care unit admission (ICU) in case of pulmonary edema or other conditions not allowing a safe and fast post-operative orotracheal extubation (Figure 5).
When performing VV ECMO assisted carinal pneumonectomy, the ECMO blood flow rate need to be modulated to maintain arterial oxygen saturation (SaO2) at least at 85–95% (23).
In fact, if ECMO circuit flow is too low, a significant blood flow would pass through the non-ventilated lung, thus resulting in upper body hypo-oxygenation with potential coronary and brain damages, the so called “Harlequin” syndrome (24,25).
The correct placement of cannulas is of paramount importance for ECMO efficacy: percutaneous cannulations should be performed with Seldinger’s technique; in case of vessel calcifications or previous vascular surgery cannulation can be extremely difficult or sometimes impossible. In these cases, targeting different vessels should be considered, if feasible, or open surgical cannulation (26). Continue surveillance of limb perfusion is mandatory to prevent tremendous ischemic complications that can be prevented or treated with the insertion of a distal perfusion catheter (27).
Correct cannula placement is crucial as well as its fixation in the appropriate position, in particular in long-lasting procedure (Figure 6).
During VV ECMO impaired drainage of a venous cannula may happen, in particular when the distal part of the cannulas are too close to each other, thus causing short circuit; in this case, in fact, arterialized blood flows towards the tip of the draining cannula instead of being directed to the pulmonary artery, thus causing a “recirculating” effect. This may happen even when two femoral cannulas are used (26).
During VA ECMO the “Harlequin syndrome” may be observed, as described above; in this case the native cardiac flows beats against the pumped blood in the aortic arch region: as a consequence, the coronary arteries and, in part, the supra aortic vessels receive hypoxic blood with heart and brain hypo perfusion. Consequently, upper extremity cyanosis is observed and a rapid repositioning of the arterial cannula in to right subclavian artery or aorta need to be accomplished (26).
Conclusions
TSP is a complex thoracic procedure indicated—most of times—when the main carina is infiltrated by tumour of the right or left main bronchus. Airway reconstruction is usually performed by an end-to-end tracheo-bronchial anastomosis with CFV with or without jet ventilation support. ECMO represents an excellent alternative to CFV during TSP, providing tissues oxygenation and CO2 removal, without disturbing lines or tubes in the surgical field, when performed with peripheral cannulation. It requires a skilled multidisciplinary team work to prevent or face major complications potentially arising during or shortly after thoracic resection: it is therefore recommended to high-volume specialized centres with clinical experience of cardiorespiratory support and extended oncologic lung surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Shanghai Chest for the series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.10.06). The series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” was commissioned by the editorial office without any funding or sponsorship. FP serves as an unpaid editorial board member of Shanghai Chest from Jun 2018 to May 2020. LS served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Shanghai Chest from Aug 2019 to Jul 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Spaggiari L, Petrella F, Galetta D. Carinal resection. Multimed Man Cardiothorac Surg 2012;2012:mms001 [Crossref] [PubMed]
- Weder W, Inci I. Carinal resection and sleeve pneumonectomy. Thorac Surg Clin 2014;24:77-83. [Crossref] [PubMed]
- Porhanov VA, Poliakov IS, Selvaschuk AP, et al. Indications and results of sleeve carinal resection. Eur J Cardiothorac Surg 2002;22:685-94. [Crossref] [PubMed]
- Rosskopfova P, Perentes JY, Ris HB, et al. Extracorporeal support for pulmonary resection: current indications and results. World J Surg Oncol 2016;14:25. [Crossref] [PubMed]
- Mitchell JD. Carinal resection and reconstruction. Chest Surg Clin N Am 2003;13:315-29. [Crossref] [PubMed]
- Alifano M, Regnard JF. Sleeve pneumonectomy. Multimed Man Cardiothorac Surg 2007;2007:mmcts.2006.002113.
- Deslauriers J, Gregoire J, Jacques LF, et al. Sleeve pneumonectomy. Thorac Surg Clin 2004;14:183-90. [Crossref] [PubMed]
- Rea F, Marulli G, Schiavon M, et al. Tracheal sleeve pneumonectomy for non-small cell lung cancer (NSCLC): Short and long-term results in a single institution. Lung Cancer 2008;61:202-8. [Crossref] [PubMed]
- Lanuti M, Mathisen DJ. Carinal resection. Thorac Surg Clin 2004;14:199-209. [Crossref] [PubMed]
- Grillo HC. Carinal reconstruction. Ann Thorac Surg 1982;34:356-73. [Crossref] [PubMed]
- Maeda M, Nakamoto K, Tsubota N, et al. Operative approaches for left-sided carinoplasty. Ann Thorac Surg 1993;56:441-5; discussion 445-6. [Crossref] [PubMed]
- Perelman MI. Surgery of the Trachea. Moscow: Mir, 1976.
- Peng G, Cui F, Ang KL, et al. Non-intubated combined with video assisted thoracoscopic in carinal reconstruction. J Thorac Dis 2016;8:586-93. [Crossref] [PubMed]
- Schweiger T, Klepetko W, Hoetzenecker K. Awake minimal invasive carinal resection-tightrope walking in thoracic surgery? J Thorac Dis 2017;9:3667-9. [Crossref] [PubMed]
- Aigner C, Wisser W, Taghavi S, et al. Institutional experience with extracorporeal membrane oxygenation in lung transplantation. Eur J Cardiothorac Surg 2007;31:468-73; discussion 473-4. [Crossref] [PubMed]
- Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol 2014;63:2769-78. [Crossref] [PubMed]
- Voelckel W, Wenzel V, Rieger M, et al. Temporary extracorporeal membrane oxygenation in the treatment of acute traumatic lung injury. Can J Anaesth 1998;45:1097-102. [Crossref] [PubMed]
- Woods FM, Neptune WB, Palatchi A. Resection of the carina and main-stem bronchi with the use of extracorporeal circulation N Engl J Med 1961;264:492-4. [Crossref] [PubMed]
- Petrella F, Salvi L, Venturino M, et al. Small bore eco guided femoral percutaneous cannulation. Asvide 2019;6:325. Available online: http://www.asvide.com/watch/33010
- Petrella F, Salvi L, Venturino M, et al. Large bore femoral percutaneous cannulation. Asvide 2019;6:326. Available online: http://www.asvide.com/watch/33012
- Brutel de la Rivière A, Knaepen P, Van Swieten H, et al. Concomitant open heart surgery and pulmonary resection for lung cancer. Eur J Cardiothorac Surg 1995;9:310-3; discussion 313-4. [Crossref] [PubMed]
- Petrella F (Principal Investigator). Circulating Tumor Cells (CTC) Before and After Thoracic Resection With and Without Intraoperative Use of ExtraCorporeal Membrane Oxygenator(ECMO) or Cardio Pulmonary By Pass (CPB). Available online: https://clinicaltrials.gov/ct2/show/NCT04048512?term=CTC%2C+ECMO&rank=1. last accessed Sep, 2019.
- Smith IJ, Sidebotham DA, McGeorge AD. Use of Extracorporeal Membrane Oxygenation during Resection of Tracheal Papillomatosis. Anesthesiology 2009;110:427-9. [PubMed]
- Beca J, Wilcox T, Hall R. Mechanical Cardiac Support, Cardiothoracic Critical Care. In: Sidebotham D, McKee A, Gillham M, et al. editors. Cardiothoracic Critical Care. 1st edition. Philadelphia: Butterworth-Heinmann Elsevier, 2007:672.
- Lei J, Su K, Li XF, et al. ECMO-assisted carinal resection and reconstruction after left pneumonectomy. J Cardiothorac Surg 2010;5:89. [Crossref] [PubMed]
- Rupprecht L, Lunz D, Philipp A. at al. Pitfalls in percutaneous ECMO cannulation. Heart Lung Vessel 2015;7:320-6. [PubMed]
- Avalli L, Sangalli F, Migliari M, et al. Early vascular complications after percutaneous cannulation for Extracorporeal Membrane Oxygenation for cardiac assist. Minerva Anestesiol 2016;82:36-43. [PubMed]
Cite this article as: Petrella F, Salvi L, Venturino M, Alamanni F, Spaggiari L. Veno-venous extracorporeal membrane oxygenation tracheal sleeve pneumonectomy. Shanghai Chest 2020;4:7.