
Percutaneous dilational tracheostomy: current techniques and evidence of safety
Introduction
References to tracheostomy can be found in literature dating back to the Egyptians. It is a technique which consists of creating an opening in the anterior wall of trachea. Jackson (1) is the first one accredited for describing the surgical tracheostomy (ST) technique in detail and that was the one which was routinely being followed for over half a century. The more modern method of percutaneous dilational technology (PDT) was described by Ciaglia et al. (2) formally in 1985. Since then, many renditions of Ciaglia’s techniques have come to forefront, but none has been as popular as the original. In the carefully-selected patient population PDT has a favorable safety profile and indeed carries distinct advantages over ST (3-6). As more intensivists and interventional pulmonologists become familiar with performing PDT, the use and applications of the procedure are likely to increase.
Procedural techniques
Patient selection is the key while deciding between ST and PDT (7). The contraindications of past such as thrombocytopenia, ongoing antiplatelet therapy, distorted neck anatomy due to body habitus, previous tracheostomies and malignancy are relative in hand of physician experienced with PDT. if a physician is still gaining experience in terms of PDT numbers then in scenarios mentioned earlier ST would be a better choice. Because PDT is an elective procedure, ideally it should not be performed in unstable patient population. In addition, if the appropriate anatomy for performing the procedure cannot be readily palpated at the bedside, ST should be considered instead. ST would also be a logical choice when large or arterial vessels are identified during survey ultrasonogram of the neck.
Currently, the modified Ciaglia method described by Bewsher et al. (8) is the most popular technique for performing PDT (9). Proper positioning of the patient is of paramount importance before starting the procedure and sedatives, analgesics along with neuromuscular agents are routinely used to achieve this target while keeping patient comfort in mind as well (7). The patient’s neck should be extended as much as possible by placing a rolled towel or something similar between the shoulder blades. This is a sterile procedure and standard surgical sterilizations protocols should be observed. Patient’s pre-oxygenation is attempted by turning the FIO2 to 100%.
The site of incision is generally 2 fingerbreadths above the sternal notch or 1 fingerbreadth below the cricothyroid membrane. Vertical midline incision is made to minimize the risk of bleeding or damage to the surrounding anatomical structures. As tracheal stenosis due to injury to the cricoid cartilage or first tracheal ring has been reported, it is prudent to select a site between the 2nd and 3rd tracheal rings to avoid this complication (10). Lidocaine with norepinephrine is used as local anesthetic prior to beginning any kind of instrumentation. The endotracheal tube (ETT) cuff is deflated, and the tube is retracted such that the cuff is immediately below the vocal cords. If bronchoscopy is used, the tube can be withdrawn under bronchoscopic guidance. The trachea is punctured between the 1st and 4th tracheal rings; the light source of bronchoscope, if used, can assist in identifying needle puncture site by serving as a guide. Needle puncture is followed by insertion of introducer sheath through which the guidewire is inserted and directed caudally into the distal airways. Introducer sheath is subsequently removed, and dilation process is then begun.
The originally-described method relies on application of a series of progressively-enlarging dilators; however, since Ciaglia’s original description, many commercial kits have become available which have just one uniquely style dilator for progressive dilation (Figure 1). In this single-dilator technique, the trachea is dilated first by using the short punch dilator followed by the conical dilator (8). The tracheostomy tube is then loaded on the introducer dilator and passed into the trachea through the dilated stoma. Alternate techniques include the use of dilating forceps (11,12), translaryngeal “pull” technique (13), screw-type rotational dilator technique (14), and balloon dilator technique (15,16). A comparison of each technique is shown in Table 1.
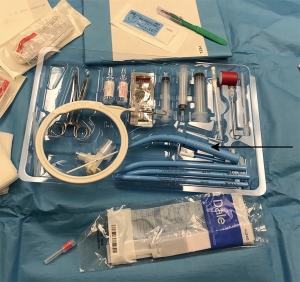
Table 1
| Technique | Describing publication | Description | Safety |
|---|---|---|---|
| Ciaglia multiple-dilator technique | Ciaglia et al. | Multiple dilators of increasing size passed over a wire to create a tract through which tracheostomy tube is placed | Appears to be preferred technique |
| Single dilator technique | Bewsher et al. | Single conical dilator replaces multiple dilators and progressively dilates tract | Limited evidence but appears equivalent to multiple dilator technique |
| Rotational dilator technique | Frova and Quintel | Single screw-type rotating dilator replaces multiple dilators and progressively dilates tract | Higher rate of procedure failure |
| Dilating forceps technique | Griggs et al. | Special dilating forceps are passed over a wire and used to dilate tract | Higher rate of major bleeding and perioperative complications |
| Balloon dilator technique | Zgoda and Berger | Dilating balloon is passed over a wire and inflated to dilate tract | Higher rate of minor complications |
| Trans-laryngeal technique | Fantoni and Ripamonti | Wire passed retrograde through trachea to exit the mouth; tracheostomy tube is attached and pulled through the mouth and out through the tracheal wall | Higher rate of procedure failure and major complications |
Complications of PDT
Early complications of PDT described in the literature include creation of a false passage in the mediastinum, inadvertent loss of airway via extubation or decannulation, posterior membrane injury, pneumothorax, hypoxemia, and bleeding (17-19), with most of these complications being minor in nature. The most commonly described serious complication has been false passage, occurring in 13 reported cases and resulting in one death (18,19). It is notable that in the case series by Kost (17), no cases of false passage occurred, underscoring a potential advantage to bronchoscopic guidance. Inadvertent extubation, while theoretically a life-threatening complication, has generally been manageable via rapid re-intubation prior to continuing the procedure (17,19); no deaths were reported due to this complication. It is notable that inadvertent decannulation and bleeding, particularly bleeding requiring transfusion, are both unusual complications; Moe et al. (18) posit that this is related to the less extensive dissection and snug fit of the tracheostomy tube compared to ST.
Late complications of PDT include peristomal infection, subglottic stenosis, and excessive peristomal granulation tissue (17-19). Two cases of tracheoesophageal fistula were also seen (18,19). Most cases of peristomal infection have involved peristomal cellulitis and were adequately treated with antibiotics and local wound care; however, Kost describe a case of necrotizing cellulitis requiring debridement and resulting in loss of two tracheal rings (17); interestingly, this patient was still eventually successfully decannulated. Tracheal stenosis has, fortunately, been an uncommon complication, and symptomatic cases are only discussed in the series by Hill et al. (19). The treatments used for symptomatic stenoses is not described except for one case in which the patient underwent laser fulguration. Fortunately, the feared complication of tracheo-innominate fistula (20) was not observed in any of these three large series.
Procedural adjuncts
As patient safety is paramount for all medical and surgical procedures, multiple bedside technologies have been used with the goal of improving safety of the PDT procedure. Among these, the most common are bronchoscopy, bedside ultrasonography, and laryngeal mask airway (LMA) placement (7). The literature regarding each of these adjuncts is reviewed below.
Bronchoscopy
The primary rationale for using bronchoscopy during PDT is to provide direct visualization of needle puncture site, thus reducing the chances of damage to posterior tracheal wall from the needle or dilators. Proper placement of the tracheostomy tube can also be ascertained. When compared with historical controls, patients undergoing bronchoscopy-assisted PDT did show a favorable rate of complications with only 3/500 having superficial damage to the posterior membrane and no episodes of pneumothorax or false passage (17); however, in the largest study directly comparing standard PDT to bronchoscopy-assisted PDT, the addition of bronchoscopy did not reduce the rate of complications and was associated with increased operational costs as well as repair costs from damaged bronchoscopes (21). In addition, a small study showed that bronchoscopy-assisted PDT resulted in a greater degree of hypercarbia related to procedure time than for PDT without bronchoscopy (22); however, there were no observed complications in this series. Further supporting the safety of bronchoscopy is the observation that even patients requiring high-frequency ventilation have shown stable oxygen requirements at 1 hour and 24-hour intervals after bronchoscopy was performed as part of PDT (23). Thus, while superiority of bronchoscopy-guided PDT has not been proven when compared to standard PDT, bronchoscopy is still a reasonable adjunct for performance of PDT.
Ultrasonography
The rationale for using bedside ultrasonography is two-fold; ultrasonography can confirm the clinician’s estimation of the underlying anatomy and guide the selection of an appropriate site, and it can reveal the presence of blood vessels that would increase the risk of procedural bleeding. Furthermore, ultrasonography can assist in gauging the depth of subcutaneous tissue leading to the anterior tracheal wall, providing a rough estimate to the operator about the needle depth for reaching from skin to trachea. Compared with standard PDT, ultrasound-guided PDT has in two comparative studies shown an improvement in first-pass success and in improving puncture accuracy (24,25); a third study showed comparable complication rates to bronchoscopy-guided PDT (26). Further benefits of pre-procedure and real-time ultrasonography described in case series have included identification of aberrant vessels for pre-operative ligation and prevention of cranial malposition of tracheostomy tubes (27). Due to technological constraints ultrasonography is not a good tool for visualization of the posterior tracheal wall and bronchoscope has an advantage over former in this regard.
LMA
Withdrawal of the ETT to the level of the vocal cords, as described by Ciaglia et al. (2), carries multiple potential risks, including accidental extubation, continued leak even with the cuff inflated, and inability to visualize the tracheal anatomy through the bronchoscope due to ETT position; thus, conversion of the airway to a LMA has been suggested as a way of avoiding these complications (28). In a 2018 Cochrane review, reviewers were able to find nine studies evaluating whether an LMA carries any advantage over an ETT for PDT (29). The median procedure time was shorter by about 1.5 min in the LMA group, while the risk of procedure failure, defined as conversion to an alternate procedure or abandonment of PDT, was about 3 times greater in the LMA group, with most failures resulted in completing the PDT procedure with an ETT. There was no significant difference in all-cause mortality, procedure-related mortality, or significant adverse events between the two groups. Evidence for all outcomes was of low to very low quality. At this point, there is insufficient evidence to recommend conversion to LMA for the purpose of PDT, although in patients where the surgical site cannot be adequately visualized through an ETT an LMA may still serve as a useful adjunct.
Comparison with ST
Multiple authors have performed systematic reviews of available literature to answer the question of whether PDT is a safe alternative to ST for patients needing long-term airway management (3-5,30). Early reviews were mixed; while Freeman et al. (3) and Delaney et al. (4) find a significant decrease in stoma infections with PDT compared with ST and a decrease in bleeding and mortality with PDT compared with ST in the operating room, Higgins and Punthakee (30) find a concurrent increase in the risk of decannulation and obstruction with PDT compared to ST. A later systematic review by Putensen et al. (5) including techniques not previously available confirms the earlier findings of Freeman et al and Delaney et al but also finds an increased risk of difficult placement with PDT compared with ST. Furthermore, Putensen et al. fail to find an increased risk of late tracheal stenosis.
A few points regarding these findings merit further discussion. Regarding the increased risk of tracheostomy obstruction found by Higgins et al. (30), the authors posit that the difference “likely relates to the fact that the open technique allows the insertion of a tracheotomy tube with an inner and outer cannula that facilitates nursing.” With modern kits this point is irrelevant as modern PDT kits allow insertion of a tracheostomy with inner cannula. Both Delaney et al. (4) and Higgins et al. (30) note a particular difference in complication rates with ST is performed in the operating room. Studies have shown that transport-related mishaps occur with significant frequency (31,32). Although Putensen et al. (5) fail to show a difference between ST performed in the operating room and at the bedside, the possibility of transport-related patient complications must be considered.
In the most recent, most comprehensive review to date performed for the Cochrane Collaboration, Brass et al. (6) find moderate-quality evidence that PDT compared with ST reduces the risk of stoma infection with a relative risk of 0.24 (95% CI, 0.15–0.37). They also find low-quality evidence of a reduced risk of unfavorable scarring, although the wide confidence interval for this outcome precludes an estimate of the magnitude of effect. The reviewers find no difference in the rate of death, intraoperative or postoperative serious complications, major bleeding, or tube occlusion/decannulation.
Regarding specific PDT methods, a meta-analysis by Cabrini et al. (33) reveals a higher rate of procedure failure with the translaryngeal technique and rotational dilator technique and a higher rate of major complications with the translaryngeal technique when compared with the Ciaglia technique. The analysis also reveals a higher rate of minor complications with the dilating forceps technique and the balloon dilation technique when compared with the Ciaglia technique. While Cabrini et al only find sufficient evidence to recommend against the translaryngeal and rotational dilator techniques, Putensen et al. (5) pool the Ciaglia and modified Ciaglia techniques together and find an increased risk of major bleeding with the dilating forceps technique. Furthermore, Brass et al. (6) find a significantly increased risk of perioperative complications with the dilating forceps technique compared to the Ciaglia technique. Taken as a whole, the data suggest that either the Ciaglia technique or its single-dilator modification may be preferable to other methods of performing PDT.
The issue of procedural cost also deserves special mention. Moe et al. (18) suggest that the cost of PDT compared to ST may be greater due to the need for bronchoscopic equipment; the authors, however, base their data on costs in Switzerland with a ST kit immediately available for every case. In contrast, Higgins et al. (30) find PDT to cost, on average, $459 less than ST among studies that mention cost. A United States-based study by Freeman et al. (34) deserves special mention. The authors, who routinely use bronchoscopic guidance for PDT cases in the study, find a cost difference of $1,569 in favor of PDT compared with ST. Thus, while the economic analysis seems to be highly dependent on local factors, in some regions, PDT may provide significant cost savings over ST.
Conclusions
PDT has emerged as a useful alternative to ST in critically ill patients needing long-term mechanical ventilation or airway support. In patients with non-emergent needs and readily-palpable tracheal anatomy, PDT can be easily performed at the bedside by intensive care staff, obviating the need for patient transport and operating room time. While procedural adjuncts have been described, none has been definitively shown to offer a measurable benefit, and it is reasonable to proceed without these adjuncts if they are not available. The Ciaglia and modified Ciaglia techniques appear to be the preferred methods of placement. In appropriately-selected patients, the safety profile of PDT compares favorably with ST and may offer a cost savings depending on local economic factors.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Douglas Kyle Hogarth and Jonathan S. Kurman) for the series “Interventional Pulmonology and Advanced Bronchoscopy” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.11.13). The series “Interventional Pulmonology and Advanced Bronchoscopy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tracheotomy Jackson C. Laryngoscope 1909;19:285-90.
- Ciaglia P, Firsching R, Syniec C. Elective percutaneous dilatational tracheostomy. A new simple bedside procedure; preliminary report. Chest 1985;87:715-9. [Crossref] [PubMed]
- Freeman BD, Isabella K, Lin N, et al. A Meta-analysis of Prospective Trials Comparing Percutaneous and Surgical Tracheostomy in Critically Ill Patients. Chest 2000;118:1412-8. [Crossref] [PubMed]
- Delaney A, Bagshaw SM, Nalos M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: a systematic review and meta-analysis. Crit Care 2006;10:R55. [Crossref] [PubMed]
- Putensen C, Theuerkauf N, Guenther U, et al. Percutaneous and surgical tracheostomy in critically ill adult patients: a meta-analysis. Crit Care 2014;18:544. [Crossref] [PubMed]
- Brass P, Hellmich M, Ladra A, et al. Percutaneous techniques versus surgical techniques for tracheostomy. Cochrane Database Syst Rev 2016;7:CD008045 [PubMed]
- Cools-Lartigue J, Aboalsaud A, et al. Evolution of percutaneous dilatational tracheostomy--a review of current techniques and their pitfalls. World J Surg 2013;37:1633-46. [Crossref] [PubMed]
- Bewsher MS, Adams AM, Clarke CW, et al. Evaluation of a new percutaneous dilatational tracheostomy set apparatus. Anaesthesia 2001;56:859-64. [Crossref] [PubMed]
- Kluge S, Baumann HJ, Maier C, et al. Tracheostomy in the intensive care unit: a nationwide survey. Anesth Analg 2008;107:1639-43. [Crossref] [PubMed]
- Sarper A, Ayten A, Eser I, et al. Tracheal stenosis after tracheostomy or intubation: review with special regard to cause and management. Tex Heart Inst J 2005;32:154-8. [PubMed]
- Schachner A, Ovil Y, Sidi J, et al. Percutaneous tracheostomy—A new method. Crit Care Med 1989;17:1052-6. [Crossref] [PubMed]
- Griggs WM, Worthley LI, Gilligan JE, et al. A simple percutaneous tracheostomy technique. Surg Gynecol Obstet 1990;170:543-5. [PubMed]
- Fantoni A, Ripamonti D. A non-derivative, non-surgical tracheostomy: the translaryngeal method. Intensive Care Med 1997;23:386-92. [Crossref] [PubMed]
- Frova G, Quintel M. A new simple method for percutaneous tracheostomy: controlled rotating dilation. A preliminary report. Intensive Care Med 2002;28:299-303. [Crossref] [PubMed]
- Zgoda MA, Berger R. Balloon-facilitated percutaneous dilational tracheostomy tube placement: preliminary report of a novel technique. Chest 2005;128:3688-90. [Crossref] [PubMed]
- Cianchi G, Zagli G, Bonizzoli M, et al. Comparison between single-step and balloon dilatational tracheostomy in intensive care unit: a single-centre, randomized controlled study. Br J Anaesth 2010;104:728-32. [Crossref] [PubMed]
- Kost KM. Endoscopic percutaneous dilatational tracheotomy: a prospective evaluation of 500 consecutive cases. Laryngoscope 2005;115:1-30. [Crossref] [PubMed]
- Moe KS, Stoeckli SJ, Schmid S, et al. Percutaneous tracheostomy: a comprehensive evaluation. Ann Otol Rhinol Laryngol 1999;108:384-91. [Crossref] [PubMed]
- Hill BB, Zweng TN, Maley RH, et al. Percutaneous dilational tracheostomy: report of 356 cases. J Trauma 1996;41:238-43; discussion 243-4. [Crossref] [PubMed]
- Mulder DS, Rubush JL. Complications of tracheostomy: relationship to long term ventilatory assistance. J Trauma 1969;9:389-402. [Crossref] [PubMed]
- Jackson LS, Davis JW, Kaups KL, et al. Percutaneous tracheostomy: to bronch or not to bronch--that is the question. J Trauma 2011;71:1553-6. [Crossref] [PubMed]
- Reilly PM, Sing RF, Giberson FA, et al. Hypercarbia during tracheostomy: a comparison of percutaneous endoscopic, percutaneous Doppler, and standard surgical tracheostomy. Intensive Care Med 1997;23:859-64. [Crossref] [PubMed]
- Shah S, Morgan P. Percutaneous dilation tracheostomy during high-frequency oscillatory ventilation. Crit Care Med 2002;30:1762-4. [Crossref] [PubMed]
- Dinh VA, Farshidpanah S, Lu S, et al. Real-time Sonographically Guided Percutaneous Dilatational Tracheostomy Using a Long-Axis Approach Compared to the Landmark Technique. J Ultrasound Med 2014;33:1407-15. [Crossref] [PubMed]
- Rudas M, Seppelt I, Herkes R, et al. Traditional landmark versus ultrasound guided tracheal puncture during percutaneous dilatational tracheostomy in adult intensive care patients: a randomised controlled trial. Crit Care 2014;18:514. [Crossref] [PubMed]
- Gobatto AL, Besen BA, Tierno PF, et al. Comparison between ultrasound- and bronchoscopy-guided percutaneous dilational tracheostomy in critically ill patients: a retrospective cohort study. J Crit Care 2015;30:220.e13-7. [Crossref] [PubMed]
- Rudas M, Seppelt I. Safety and efficacy of ultrasonography before and during percutaneous dilatational tracheostomy in adult patients: a systematic review. Crit Care Resusc 2012;14:297-301. [PubMed]
- Dexter TJ. The Laryngeal Mask Airway: A Method to Improve Visualisation of the Trachea and Larynx during Fibreoptic Assisted Percutaneous Tracheostomy. Anaesth Intensive Care 1994;22:35-9. [Crossref] [PubMed]
- Strametz R, Bergold MN, Weberschock T. Laryngeal mask airway versus endotracheal tube for percutaneous dilatational tracheostomy in critically ill adults. Cochrane Database Syst Rev 2018;11:CD009901 [PubMed]
- Higgins KM, Punthakee X. Meta-analysis comparison of open versus percutaneous tracheostomy. Laryngoscope 2007;117:447-54. [Crossref] [PubMed]
- Smith I, Fleming S, Cernaianu A. Mishaps during transport from the intensive care unit. Crit Care Med 1990;18:278-81. [Crossref] [PubMed]
- Venkategowda PM, Rao SM, Mutkule DP, et al. Unexpected events occurring during the intra-hospital transport of critically ill ICU patients. Indian J Crit Care Med 2014;18:354-7. [Crossref] [PubMed]
- Cabrini L, Monti G, Landoni G, et al. Percutaneous tracheostomy, a systematic review. Acta Anaesthesiol Scand 2012;56:270-81. [Crossref] [PubMed]
- Freeman BD, Isabella K, Cobb JP, et al. A prospective, randomized study comparing percutaneous with surgical tracheostomy in critically ill patients. Crit Care Med 2001;29:926-30. [Crossref] [PubMed]
Cite this article as: Ghori UK, Chambers DM. Percutaneous dilational tracheostomy: current techniques and evidence of safety. Shanghai Chest 2020;4:22.


