
Endobronchial ultrasound needles: does size matter?
Introduction
Transbronchial needle aspiration (TBNA) was first reported in 1949 (1), but it was not until 1978 that Wang et al. popularized the conventional TBNA technique for the diagnosis of paratracheal tumors with the use of a 23-gauge (G) esophageal varices needle. In this small case series, tissue diagnosis was established in 3 of the 5 patients, including one with small cell lung cancer (2). A flexible needle was specifically designed for TBNA in 1983 (3), and its efficacy in diagnosing sarcoidosis was demonstrated in 1989 (4). The advent of endobronchial ultrasound-guided (EBUS) TBNA has revolutionized mediastinal sampling over the past 15 years and is now recognized as a first-line diagnostic modality in the evaluation of patients with mediastinal and hilar lesions/lymphadenopathy (5-7). EBUS-TBNA is frequently utilized in the staging of suspected or established lung cancer, and it is additionally used for the diagnosis of unexplained lymphadenopathy and suspected granulomatous diseases or lymphoproliferative disorders (8-10).
Several needle sizes are commercially available, including 25-, 22-, 21-, and 19G, and are made of various materials (Table 1). Notably, professional guidelines are equivocal regarding the preferred needle size to be used for lymph node sampling, and there is conflicting data regarding the efficacy of differing needle sizes when it comes to diagnostic yield and adequate sampling. For example, the American College of Chest Physicians Guidelines from 2016 gave a Grade 1C recommendation to use “either a 21- or 22-G needle” in patients undergoing EBUS-TBNA (11), and there is no expert consensus regarding the use of other needle sizes, including 25 and 19G. The professed advantages of the 25G needle include better penetration, reduced specimen contamination with blood, and a decreased deformity of the needle (10). Unfortunately, the data supporting its use is sparse. Initial studies of the conventional 19G needle suggested increased tissue sampling size, which may be needed for histologic evaluation and advanced molecular testing (12,13). In general, it is thought that the 22- and 21G needles are sufficient for cytology, whereas a 19G needle may be used to obtain a tissue core for histopathologic evaluation (11). However, a direct comparison of all needle sizes has not been performed.
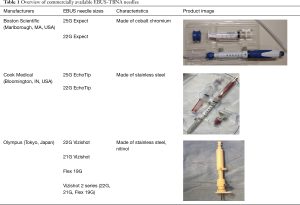
Full table
Recently, a flexible 19G EBUS-TBNA needle (Flex 19G; Olympus Respiratory America, Redmond, WA) became available for use, and there have since been several feasibility trials and prospective studies comparing this needle with smaller commercially available needles. A retrospective analysis by Tremblay et al. showed the Flex 19G needle to have high diagnostic rates for all indications (8). Furthermore, reports from Kinoshita et al. and Jones et al. suggest that the Flex 19G needle improves histopathologic analysis, which would allow for better subclassification of disease (14,15). Despite the theoretical advantages of a larger needle providing more tissue, a review of all the studies evaluating EBUS-TBNA needle size has not been thoroughly conducted to date. Herein, we examine the effect of needle size on overall diagnostic yield, outcomes in patients with sarcoidosis and lymphoproliferative disorders, and purported advantages and disadvantages of various EBUS-TBNA needle sizes.
Does needle size affect overall diagnostic yield and sampling adequacy?
There have been numerous studies comparing the quantity and quality of tissue samples obtained by different EBUS-TBNA needle gauges; however, the results from these studies are conflicting. Direct comparisons between studies are limited due to differences in procedural technique, use of rapid on-site cytopathologic evaluation (ROSE), and determination of adequate sampling (i.e., defined by ROSE, cytologic analysis, or histopathology). In addition, there are no studies comparing all sizes together, either prospectively or retrospectively.
Most commonly, the efficacy of the 21G needle compared to the 22G EBUS-TBNA needle has been investigated. Nakajima et al. compared tissue obtained from 45 lesions with both needle sizes, and sampling with the 22G was performed prior to use of the 21G needle. There was no difference in the cytopathologic diagnostic yield between groups, but there was improved histologic preservation in the 21G arm as well as increased quantity of tumor cells (16). Saji et al. also compared sampling yield and adequacy between the 21- and 22G needles; however, patients were assigned to either group in a non-randomized fashion. Overall, the combined cytologic and histopathologic diagnosis and sampling adequacy was higher in the 21G group. Notably, the rate of diagnosis with the 22G group was much lower than prior studies, which may be attributable to population bias, a lack of ROSE, and the low number of needle passes per patient and per lesion (less than 2) (17).
In a study by Oki et al., patients were prospectively randomized to undergo EBUS-TBNA with a 21- or 22G needle, with at least 2 punctures performed per target lesion. There was no significant difference in the histologic diagnostic yield or sampling adequacy between groups (18). Yarmus et al. conducted a retrospective review of 1,235 patients from an electronic registry including 6 centers in the United States. EBUS-TBNA technique at each of the centers was not highlighted but was determined at the discretion of the bronchoscopist. Again, there was no difference in diagnostic yield or sample adequacy per lymph node between needles sizes. However, with the availability of ROSE, it may be noted that there were a fewer number of needle passes per lymph node station in the 21G arm (19). An additional retrospective study by Jeyabalan et al. compared 21- and 22G samples in 303 patients referred to a university hospital in southwest England. The use of either needle was determined by the bronchoscopist, but histopathologists were blinded to the needle size. Ultimately, they concluded that there was no difference in the diagnostic yield in patients with malignancy; however, there was noted improved characterization of sarcoidosis in the 21G needle arm (20) (Table 2).

Full table
More recently, the efficacy of a flexible 19-gauge EBUS-TBNA needle has been investigated, with the thought that the larger needle size would increase the amount of diagnostic tissue obtained. Chaddha et al. prospectively compared the diagnostic yield of EBUS-TBNA in 27 patients with samples collected in an alternating manner, with both the Flex 19G and 22G needles, and kept in separate cell blocks. Overall, they found that there was no significant difference in diagnostic yield; however, the 19G needle yielded samples that were significantly less adequate (46% vs. 73%, P<0.001) and bloodier (59% vs. 19%, P<0.001) compared to the 22G needle passes. Adequacy was based on ROSE, defined by containing lesion cells, at least 40 lymphocytes/hpf, tangible body macrophages, or anthracotic pigment-laden macrophages (21). Studies by Wolters et al., Jones et al., and Dooms et al. again did not show any significant difference in the diagnostic yield of specimens obtained with the 19G needle compared to 21- or 22G needles (15,22,23). In a prospective observational study by Garrison et al., additional passes performed with a flexible 19G needle, after sampling with a 22G needle, revealed no difference in diagnostic yield when comparing each needle alone. However, sampling with the 19G needle in addition to the 22G needle resulted in a significantly increased diagnostic yield compared to the use of the 22G needle alone (24) (Table 3).
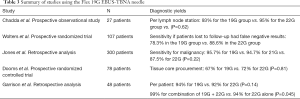
Full table
Lastly, the data comparing a 25G needle size is limited, with several studies looking at the use of this needle size in endoscopic ultrasound-guided biopsy of gastrointestinal malignancies. A retrospective study by Di Felice et al. comparing the use of 25- vs. 22G needles for EBUS-TBNA of 158 lymph nodes showed comparable specimen adequacy (92.4% vs. 92.4%, P=1) and diagnostic accuracy (98.2% vs. 94.3%, P=0.7) (25).
Although there have been a few conflicting results, a review of prominent relevant studies has consistently shown that the diagnostic yield is similar between all EBUS-TBNA needle sizes when assessing all sampled lesions together. Interestingly, one study revealed improved diagnostic yield using a 19G and 22G needle in alternating succession, which may derive the benefits of both a larger and smaller needle gauge. It is important to note, however, that other clinical endpoints including adequacy of histopathology samples, characterization of malignant subtypes and granulomatous disease, and the size of a tissue core may be optimized with specific needle gauges.
Does needle size affect diagnostic yield for sarcoidosis?
The ACCP guidelines give a Grade 1C recommendation that EBUS-TBNA be used for diagnosis in patients with suspected sarcoidosis with mediastinal or hilar adenopathy, without any recommendations regarding needle size (11). These expert consensus guidelines as well as a meta-analysis of 21 studies conducted by Agarwal et al. have cited a pooled diagnostic accuracy of 79% in patients with sarcoidosis (11,26). Interestingly, in the latter study, the diagnostic yield was significantly higher in studies using a 19G histology needle versus those using smaller gauges (26). A study by Tyan et al. also revealed an absolute higher diagnostic yield of 93% in a subgroup analysis of patients with sarcoidosis when using a 19G needle; however, this was not compared with other needle sizes (27).
A subgroup analysis of benign lesions in Jeyabalan et al. demonstrated superior characterization (especially for sarcoidosis) in 83% of 21G tissue samples compared to 60% of 22G tissue samples (P<0.01) (20). Similarly, in a retrospective analysis examining all three needle sizes by Jones et al., there was a higher rate of sub-characterization with the 19G needle in benign disease, especially in those with sarcoidosis (15). In contrast, a prospective randomized controlled trial by Muthu et al. comparing 21G with 22G needles revealed a lack of difference in yield in patients with sarcoidosis (28). Pickering et al. revealed discrepancies in the histologic diagnosis of granulomatous disease between a flexible 19G and 21G needle utilized in alternating fashion, and one patient would not have been diagnosed if only the 19G needle was used. Regardless, there were only a total of 10 patients ultimately diagnosed with sarcoidosis, and this sample size was too small to draw any conclusions (29).
In conclusion, there has not been a large prospective randomized controlled trial comparing the yield between all needle groups in the sarcoidosis population. However, subgroup analyses of available studies suggest that the use of a 19G needle may be of use, especially when trying to distinguish sarcoidosis from other benign pathologies. Large prospective, randomized controlled trials are needed, especially evaluating the newer flexible 19G needle. Regardless, the diagnostic yield for sarcoidosis can be optimized by utilizing a multi-modality approach: endobronchial biopsies, transbronchial biopsies, and EBUS-TBNA (30,31).
Does needle size affect diagnostic yield for lymphoma?
EBUS-TBNA is now generally accepted as an initial diagnostic modality in patients with suspected lymphoma. The ACCP guidelines provide an ungraded consensus-based statement supporting its initial use for this condition (11). However, it is important to highlight that both non-Hodgkin and Hodgkin’s lymphoma require evaluation of cell morphology, immunophenotyping and tissue architecture analysis to guide treatment, frequently necessitating larger histopathologic samples in addition to cytology. In the ACCP guidelines, a review of five retrospective case studies on patients undergoing EBUS-TBNA for suspected lymphoma revealed a pooled diagnostic accuracy of 68.7% (9,32-35). Kennedy et al. and Moonim et al. reported an even higher diagnostic yield of 89–91%; although, it is important to note that some patients required additional tissue sampling for sub-classification, despite the initial sample being considered diagnostic and especially for Hodgkin’s lymphoma (33,34). The sample processing was protocolized with triaging into different cell suspensions based on the results of ROSE (34).
A recent systematic review of fourteen studies (425 participants) evaluating EBUS-TBNA for lymphoma diagnosis reported an overall sensitivity of 66.2%. Notably, when evaluating subgroups, there were no statistically significant differences in overall and new diagnosis of lymphoma between needle sizes. However, the sensitivity increased from 63% to 82% when using a 22G needle in patients with suspected lymphoma recurrence compared to using 21G needles. This review was limited by high heterogeneity among studies, and the 19G needle was not compared (36). The use of a 19G needle via endoscopic ultrasound (EUS) in a study by Yasuda et al. was very effective at diagnosing (97%) and subtyping (89%) lymphoproliferative disease, but there was no comparison arm using smaller gauge needles (37).
In a recent retrospective analysis by Grosu et al., EBUS-TBNA with the use of a 22G needle could establish a diagnosis of lymphoma in 84% of cases, with 77% of these samples subclassified by flow cytometry and immunohistochemical analysis (38). Their EBUS-TBNA technique was different prior studies, such as Iqbal et al. (32), as they performed an average of 5 passes per lymph node with a 22G needle, with performance of cell counts to obtain 1 million cells, rather than subjective estimates of visible cores. There was no comparison to other needle sizes.
Regarding subtyping, studies by Nason et al. and Ko et al. have both demonstrated success in about 70% of cases (39,40), using primarily 22G needles, but the needle size was not always recorded. All in all, there is a paucity of data comparing diagnostic yield and ability to subtype lymphoma between needle sizes, especially with the flexible 19G needle. Furthermore, subtyping may be less important in cases of recurrent disease, and a smaller needle gauge may be acceptable. Altogether, studies have shown that diagnosing and subtyping lymphoma is possible, especially with the use of ROSE, but more studies are needed to make any definitive conclusions regarding the optimal EBUS-TBNA needle size.
Are there additional benefits to certain needle sizes?
The need for increased tissue samples for immunophenotyping, molecular studies, and next-generation sequencing (NGS) raises the question as to whether the larger needle sizes provide more tissue and increase the sample adequacy.
In regard to overall tissue size obtained with the 19G needle, tissue core procurement by measuring absolute tissue area, mean cell area, and sample weight have all been investigated. A study by Dooms et al. revealed a trend towards larger tissue area with a 19G needle; however, this did not alter the diagnostic yield or the success of NGS (22). Conversely, an investigation by Pickering et al. showed that the cellular material obtained by a flexible 19G needle was significantly greater compared to a 21G needle, with a subgroup analysis revealing increased cell area in the malignant patient population. Additionally, there was about 40% more tissue obtained with each pass with the 19G needle. Enough material was obtained for all designated tumor biomarker testing requested; although, NGS adequacy was not evaluated as part of this study (29). Recently, Wolters et al. compared sample weight in EBUS-TBNA using a 19- and 22G needle, demonstrating no difference in diagnostic yield; however, there was a significantly higher number of quantified tumor cells in the 19G group. The authors noted that this could have implications for additional molecular workup of patients, but this remains to be investigated (23).
Overall, the 19G needle provides larger tissue area and cellular material; however, none of the studies that demonstrated that this has played a significant role in altering the diagnostic yield. As the molecular work-up and immunophenotyping of tumors becomes more advanced, larger tissue samples may be warranted, thus necessitating the use of a 19G EBUS-TBNA needle.
Are there any disadvantages to certain needle sizes?
In regard to adverse effects, review of all aforementioned studies did not reveal any significant difference in complications (such as significant bleeding or barotrauma) between needle sizes. In the study by Tremblay et al. examining cases of EBUS-TBNA with a flexible 19G needle, there was one noted case of significant hemoptysis (<50 mL) that was managed by reinsertion of the bronchoscope to suction retained blood in the airways, after which no active bleeding was noted. In this same study, there was one case where the operator could not penetrate the target lesion with the 19G needle, and it was sampled with a 21G needle instead (8). This may be due to the increased stiffness of the 21G needle. However, there were 2 patients in the Pickering et al. study in which the lesion could not be sampled with either the 19- or 21G needles (29).
The overall complication rate with EBUS-TBNA is low, 0.97–1.23% in some studies (41,42). The most common complications are hemorrhage, infection, and pneumothorax (42). Although not explicitly noted in the reviewed studies, there is the possibility of specific complications and technical difficulties when using certain EBUS needles. Stiffer needles, such as the 21G Vizishot, allow for smoother penetration but are not ideal for accessing difficult nodal sites (e.g., 4L and 10R). On the contrary, more flexible needles, such as the Flex 19G, facilitate sampling of more difficult to access areas. This flexibility also affects the angle of entry and trajectory as highlighted in Figure 1. Breakage of the needle has previously been reported with a rate of 0.20% (42). Such an event has occurred with the Flex 19G needle (Figure 2). Additionally, the Flex 19G needles are more costly compared to the 21- and 22G EBUS-TBNA needles.
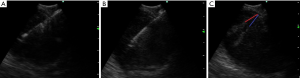
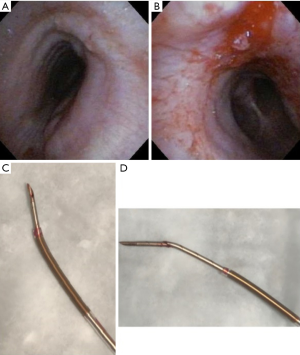
Overall, a more tailored approach to accessing individual nodes is ideal, and if possible, the use of a combination of various needle sizes and flexibility capabilities is optimal. However, given the increased cost of the newer larger EBUS needles, this may not always be possible.
Conclusions
In summary, when comparing EBUS-TBNA needle sizes, there appears to be no difference in overall diagnostic yield and tissue sampling. However, it is important to consider a more tailored approach in various subpopulations, such as patients with suspected sarcoidosis and lymphoma. If the clinical diagnosis is unclear, there is a suspicion for either granulomatous disease or lymphoma, or there is a need for increased tissue for molecular testing and immunophenotyping, it may be prudent to use a 19G needle or even two needle sizes in alternating fashion. Multiple factors should be taken into account when selecting needle size, including the accessibility of a lymph node station, EBUS scope engagement, presence of intra-nodal vessels, and desired penetrability when sampling.
All in all, more randomized prospective studies comparing the Flex 19G needle in the aforementioned subpopulations are needed. Future studies may also include newly available needles (e.g., Olympus Vizishot 2 series) and fine needle biopsy devices (e.g., Boston Scientific Acquire, Cook Medical ProCore, Medtronic SharkCore Minneapolis, MN, USA).
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Schieppati E. Mediastinal lymph node puncture through the tracheal carina. Surg Gynecol Obstet 1958;107:243-6. [PubMed]
- Wang KP, Terry P, Marsh B. Bronchoscopic needle aspiration biopsy of paratracheal tumors. Am Rev Respir Dis 1978;118:17-21. [PubMed]
- Schenk DA, Bryan CL, Bower JH, et al. Transbronchial needle aspiration in the diagnosis of bronchogenic carcinoma. Chest 1987;92:83-5. [Crossref] [PubMed]
- Wang KP, Johns CJ, Fuenning C, et al. Flexible transbronchial needle aspiration for the diagnosis of sarcoidosis. Ann Otol Rhinol Laryngol 1989;98:298-300. [Crossref] [PubMed]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [Crossref] [PubMed]
- Adams K, Shah PL, Edmonds L, et al. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009;64:757-62. [Crossref] [PubMed]
- Varela-Lema L, Fernandez-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound–transbronchial needle aspiration: a systematic review. Eur Respir J 2009;33:1156-64. [Crossref] [PubMed]
- Tremblay A, Stather DR, MacEachern P, et al. A randomized controlled trial of standard vs endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest 2009;136:340-6. [Crossref] [PubMed]
- Steinfort DP, Conron M, Tsui A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the evaluation of suspected lymphoma. J Thorac Oncol 2010;5:804-9. [Crossref] [PubMed]
- Gomez M, Silvestri GA. Endobronchial ultrasound for the diagnosis and staging of lung cancer. Proc Am Thorac Soc 2009;6:180-6. [Crossref] [PubMed]
- Wahidi MM, Herth F, Yasufuku K, et al. Technical aspects of endobronchial ultrasound-guided transbronchial needle aspiration: CHEST guideline and expert panel report. Chest 2016;149:816-35. [Crossref] [PubMed]
- Schenk DA, Chambers SL, Derdak S, et al. Comparison of the Wang 19-gauge and 22-gauge needles in the mediastinal staging of lung cancer. Am Rev Respir Dis 1993;147:1251-8. [Crossref] [PubMed]
- Ben S, Akulian J, Wang KP. Endobronchial ultrasound transbronchial needle aspiration: a hybrid method. J Thorac Dis 2015;7:S287-91. [PubMed]
- Kinoshita T, Ujiie H, Schwock J, et al. Clinical evaluation of the utility of a flexible 19-gauge EBUS-TBNA needle. J Thorac Dis 2018;10:2388-96. [Crossref] [PubMed]
- Jones RC, Bhatt N, Medford AR. The effect of 19-gauge endobronchial ultrasound-guided transbronchial needle aspiration biopsies on characterisation of malignant and benign disease. The Bristol experience. Monaldi Arch Chest Dis 2018;88:915. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Takahashi R, et al. Comparison of 21-gauge and 22-gauge aspiration needle 2 during endobronchial ultrasound-guided transbronchial needle aspiration. Respirology 2011;16:90-4. [Crossref] [PubMed]
- Saji J, Kurimoto N, Morita K, et al. Comparison of 21-gauge and 22-gauge needles for endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. J Bronchology Interv Pulmonol 2011;18:239-46. [Crossref] [PubMed]
- Oki M, Saka H, Kitagawa C, et al. Randomized study of 21-gauge versus 22-gauge endobronchial ultrasound-guided transbronchial needle aspiration needles for sampling histology specimens. J Bronchology Interv Pulmonol 2011;18:306-10. [Crossref] [PubMed]
- Yarmus LB, Akulian J, Lechtzin N, et al. Comparison of 21-gauge and 22-gauge aspiration needle in endobronchial ultrasound-guided transbronchial needle aspiration: results of the American College of Chest Physicians Quality Improvement Registry, Education, and Evaluation Registry. Chest 2013;143:1036-43. [Crossref] [PubMed]
- Jeyabalan A, Shelley-Fraser G, Medford AR. Impact of needle gauge on characterization of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) histology samples. Respirology 2014;19:735-9. [Crossref] [PubMed]
- Chaddha U, Ronaghi R, Elatre W, et al. Comparison of sample adequacy and diagnostic yield of 19-and 22-G EBUS-TBNA needles. J Bronchology Interv Pulmonol 2018;25:264-8. [Crossref] [PubMed]
- Dooms C, Vander Borght S, Yserbyt J, et al. A randomized clinical trial of Flex 19G needles versus 22G needles for endobronchial ultrasonography in suspected lung cancer. Respiration 2018;96:275-82. [Crossref] [PubMed]
- Wolters C, Darwiche K, Franzen D, et al. A Prospective, Randomized Trial for the Comparison of 19-G and 22-G Endobronchial Ultrasound-Guided Transbronchial Aspiration Needles; Introducing a Novel End Point of Sample Weight Corrected for Blood Content. Clin Lung Cancer 2019;20:e265-73. [Crossref] [PubMed]
- Garrison G, Leclair T, Balla A, et al. Use of an additional 19-G EBUS-TBNA needle increases the diagnostic yield of EBUS-TBNA. J Bronchology Interv Pulmonol 2018;25:269-73. [Crossref] [PubMed]
- Di Felice C, Young B, Matta M. Comparison of specimen adequacy and diagnostic accuracy of a 25-gauge and 22-gauge needle in endobronchial ultrasound-guided transbronchial needle aspiration. J Thorac Dis 2019;11:3643-9. [Crossref] [PubMed]
- Agarwal R, Srinivasan A, Aggarwal AN, et al. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: a systematic review and meta-analysis. Respir Med 2012;106:883-92. [Crossref] [PubMed]
- Tyan C, Patel P, Czarnecka K, et al. Flexible 19-gauge endobronchial ultrasound-guided transbronchial needle aspiration needle: first experience. Respiration 2017;94:52-7. [Crossref] [PubMed]
- Muthu V, Gupta N, Dhooria S, et al. A prospective, randomized, double-blind trial comparing the diagnostic yield of 21-and 22-gauge aspiration needles for performing endobronchial ultrasound-guided transbronchial needle aspiration in sarcoidosis. Chest 2016;149:1111-3. [Crossref] [PubMed]
- Pickering EM, Holden VK, Heath JE, et al. Tissue Acquisition During EBUS-TBNA: Comparison of Cell Blocks Obtained From a 6 19G Versus 21G Needle. J Bronchology Interv Pulmonol 2019;26:237-44. [Crossref] [PubMed]
- Goyal A, Gupta D, Agarwal R, et al. Value of different bronchoscopic sampling techniques in diagnosis of sarcoidosis: a prospective study of 151 patients. J Bronchology Interv Pulmonol 2014;21:220-6. [Crossref] [PubMed]
- Dziedzic DA, Peryt A, Orlowski T. The role of EBUS-TBNA and standard bronchoscopic modalities in the diagnosis of sarcoidosis. Clin Respir J 2017;11:58-63. [Crossref] [PubMed]
- Iqbal S, DePew ZS, Kurtin PJ, et al. Endobronchial ultrasound and lymphoproliferative disorders: a retrospective study. Ann Thorac Surg 2012;94:1830-4. [Crossref] [PubMed]
- Kennedy MP, Jimenez CA, Bruzzi JF, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thorax 2008;63:360-5. [Crossref] [PubMed]
- Moonim MT, Breen R, Fields PA, et al. Diagnosis and subtyping of de novo and relapsed mediastinal lymphomas by endobronchial ultrasound needle aspiration. Am J Respir Crit Care Med 2013;188:1216-23. [Crossref] [PubMed]
- Senturk A, Babaoglu E, Kilic H, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Asian Pac J Cancer Prev 2014;15:4169-73. [Crossref] [PubMed]
- Labarca G, Sierra-Ruiz M, Kheir F, et al. Diagnostic Accuracy of Endobronchial Ultrasound Transbronchial Needle Aspiration in Lymphoma: A Systematic Review and Meta-analysis. Ann Am Thorac Soc 2019;16:1432-9. [Crossref] [PubMed]
- Yasuda I, Goto N, Tsurumi H, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy for diagnosis of lymphoproliferative disorders: feasibility of immunohistological, flow cytometric, and cytogenetic assessments. Am J Gastroenterol 2012;107:397-404. [Crossref] [PubMed]
- Grosu HB, Iliesiu M, Caraway NP, et al. Endobronchial ultrasound–guided transbronchial needle aspiration for the diagnosis and subtyping of lymphoma. Ann Am Thorac Soc 2015;12:1336-44. [Crossref] [PubMed]
- Ko HM, da Cunha Santos G, Darling G, et al. Diagnosis and subclassification of lymphomas and non‐neoplastic lesions involving mediastinal lymph nodes using endobronchial ultrasound‐guided transbronchial needle aspiration. Diagn Cytopathol 2013;41:1023-30. [Crossref] [PubMed]
- Nason KS, Kirchner A, Schuchert MJ, et al. Endobronchial ultrasound-transbronchial needle aspiration 9 for lymphoma in patients with low suspicion for lung cancer and mediastinal lymphadenopathy. Ann Thorac Surg 2016;101:1856-63. [Crossref] [PubMed]
- Kayawake H, Chen-Yoshikawa TF, Oda H, et al. Complications of endobronchial ultrasound-guided transbronchial needle aspiration. Ann Thorac Surg 2017;104:e363-5. [Crossref] [PubMed]
- Asano F, Aoe M, Ohsaki Y, et al. Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration: a nationwide survey by the Japan Society for Respiratory Endoscopy. Respir Res 2013;14:50. [Crossref] [PubMed]
Cite this article as: Kanth S, Pickering EM, Sachdeva A, Holden VK. Endobronchial ultrasound needles: does size matter? Shanghai Chest 2020;4:24.


