Pneumonectomy for lung cancer
Introduction
Lung cancer is the leading cause of cancer-related death in the United States; each year, more than 25,000 procedures are performed to treat this disease (1). The 5-year overall survival for all patients with lung cancer is a dismal 18% (2,3). In 2019, 228,150 new cases of lung cancer were estimated to be diagnosed as well as 142,670 projected deaths from the disease (4).
Non-small cell lung cancer (NSCLC) accounts for 84% of all primary bronchogenic carcinomas. Surgical resection remains the cornerstone of treatment for early-stage and locoregionally advanced NSCLC (stages I through IIIA). Only 25% to 30% of patients present with early-stage disease, and an additional 25% to 30% of patients are eligible for surgical resection with curative intent (5). Five-year overall survival rates for patients with NSCLC who have undergone surgery range from 75% for stage IA disease to 25% for stage IIIA disease (6).
Pneumonectomy accounts for 7.5% of all major lung resections (7). Common indications for pneumonectomy for lung cancer include locally advanced, central tumors with invasion of vascular and/or bronchial structures (8-10). Although the use of pneumonectomy for N2 disease has been described, this indication remains controversial and is restricted to select patients who have undergone neoadjuvant treatment, often in the context of a clinical trial (11,12). Stage-related 5-year survival rates after pneumonectomy for NSCLC may be as high as 44% for stage I, 37.5% for stage II, and 29% for stage III disease (13-15). However, when the overall survival rates are calculated, these range from 21% to 31% (16,17).
History of pneumonectomy
In 1903, Ferdinand Sauerbruch designed a negative-pressure chamber that allowed for the performance of surgery in the pleural cavity under open pneumothorax without collapse of the lung. In 1908, in New York, Willy Meyer modified the machinery to work with both positive and negative pressures. It was not until 1931, in Berlin, that Rudolph Nissen performed the first successful pneumonectomy (18). A year later, Cameron Haight performed the first successful pneumonectomy in the Western hemisphere (19).
The first successful pneumonectomy for lung cancer was performed by Evarts Graham in 1933 and became, at that time, the definitive procedure for this disease (20). In 1938, Richard Overholt reported his experience on 22 pneumonectomies (21). Meanwhile, Clarence Crafoord published a monograph focusing on the standardization of the technical aspects of pneumonectomy, including the periscapular incision with subperiosteal resection of the fifth rib, individual vessel ligation, suture closure of the bronchus, and a new rhythmic ventilatory technique that included the use of bronchial blockers (22). Overholt described the technique of hilar dissection, emphasizing the advantage of bronchial transection before dissecting the hilar vessels; with this technique, he achieved a reported mortality for patients with lung cancer of 33% (21). Following these reports and for the next two decades, pneumonectomy became the standard treatment for lung cancer and accounted for 30% of lung resections until the 1990s (Table 1).
Table 1
| Year | Surgeon | Country | Events |
|---|---|---|---|
| 1903 | Ferdinand Sauerbruch | Germany | Designed a negative-pressure chamber that allowed for surgery in the pleural cavity |
| 1908 | Willy Meyer | US | Designed a negative and positive “differential” pressure chamber in the management of open thoracotomy |
| 1931 | Rudolph Nissen | Germany | Performed the first successful pneumonectomy for a patient with chronic empyema |
| 1932 | Cameron Haight | US | Performed the first successful pneumonectomy in the US |
| 1933 | Evarts Graham | US | Performed the first successful pneumonectomy for a patient with lung cancer |
| 1938 | Richard Overholt | US | Performed the first successful right pneumonectomy and described the technique of hilar dissection |
| 1938 | Clarence Crafoord | Sweden | Published a monograph focusing on the standardization of the technical aspects of pneumonectomy, “On the Technique of Pneumonectomy in Man” |
| 1993 | William Walker | UK | Performed the first thoracoscopic pneumonectomy |
In 1993, William Walker performed the first thoracoscopic pneumonectomy (23). The pulmonary artery and veins were divided using a linear stapler, and a clamp was preliminarily applied to the main arterial truncus to control possible bleeding. The lung was removed via minithoracotomy in the fifth intercostal space. The patient was discharged on the fifth postoperative day, without complications (23). Since then, several authors have described in detail the various options for pneumonectomy by minimally invasive approaches, with acceptable morbidity, mortality, and oncologic outcomes (24-26).
Indications for pneumonectomy
The standard indication for pneumonectomy for lung cancer is represented by an extent of disease that is not amenable to lung-sparing resection in the presence of sufficient cardiopulmonary reserve. Examples typically include centrally located tumors and interlobar invasion. In addition, the involvement of the mainstem bronchus, ipsilateral pulmonary artery, or both ipsilateral pulmonary veins, as well as the presence of synchronous ipsilateral upper and lower lobe malignancies, can often require a pneumonectomy.
Preoperative evaluation
Preresection functional assessment should include the patient’s performance status as well as a formal geriatric evaluation in the elderly patient. Cardiopulmonary reserve should be assessed by pulmonary function testing and electrocardiography along with an echocardiogram (27). Predicted postoperative forced expiratory volume in one second (FEV1) and diffusing capacity of the lungs for carbon monoxide (DLCO) should be calculated, as these have been shown to greatly influence postoperative morbidity and mortality as well as quality of life parameters (i.e., long-term dyspnea, need for permanent oxygen therapy) (28).
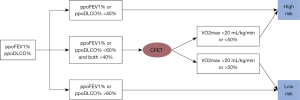
Patients with a preoperative FEV1 >2 L and predicted postoperative FEV1 >800 mL are considered to be eligible for pneumonectomy (29). In patients with borderline pulmonary parameters (FEV1 <2 L), a quantitative perfusion scan should be performed (30). A predicted postoperative FEV1 or DLCO of <40% indicates an increased risk of perioperative complications (31). In selected patients with poor performance on the stair climbing test, cardiopulmonary exercise testing should be performed (32). Simple cardiopulmonary exercise testing assessment can be in the form of stair climbing, 6- and 12-min walk tests, or the shuttle walk test. However, more commonly, cardiopulmonary exercise testing with cycloergometry is used to measure maximal oxygen consumption (VO2 max). Pneumonectomy candidates consuming <20 mL/kg/min or VO2 max <50% of predicted are at higher risk for postoperative morbidity and mortality (33). Finally, smoking cessation should be strongly encouraged, and a program should be in place to provide support with both counseling and approved pharmacologic adjuncts (Figure 1).
Clinical staging
Preresection oncologic evaluation should include a PET-CT and MRI of the brain to rule out distant metastases. Fiberoptic bronchoscopy, endobronchial ultrasound-guided biopsy, or mediastinoscopy to assess N2/3 disease should be performed (34). If indicated, an anterior mediastinotomy or thoracoscopy can be useful to determine involvement of nodal stations 5 and 6. Central tumors with concurrent pleural effusions should be subjected to thoracoscopic assessment of the pleural surfaces before committing to pneumonectomy.
Surgical decision-making: pneumonectomy versus sleeve lobectomy
Despite the high incidence of lung cancer and the number of patients with stage II and III disease, rates of pneumonectomy are declining, largely secondary to the emergence of induction therapies and the use of parenchymal-sparing resections. Sleeve resection of the bronchus, the pulmonary artery, or both has the advantage of providing complete tumor resection while avoiding pneumonectomy and has been proven to be a valid oncologic approach, as discussed below (35).
In 1947, Clement Price Thomas reported the first bronchial sleeve resection (36). In 1960, Donald Paulson presented encouraging survivals after bronchoplasty procedures performed for hilar tumors (37). Initially proposed for patients with lung cancer and poor cardiopulmonary function, sleeve lobectomy has progressively gained acceptance and is a reasonable alternative to pneumonectomy in select patients (38). The most common anatomic location for sleeve resections is the upper lobe (39-41). Sleeve lobectomy is oncologically equivalent to pneumonectomy, with comparable tumor recurrence rates. Maurizi et al. reported 5-year overall and disease-free survival for patients with centrally located NSCLC following sleeve resection of 55.1% and 62.9%, respectively (42). Abdelsattar et al. compared the results of sleeve resection (n=1,713; 7.2%) and pneumonectomy (n=22,251; 92.9%) for NSCLC in a multilevel propensity-matched observational study (43). The authors observed lower rates of 30-day (1.6% vs. 5.9%; P<0.001) and 90-day (4% vs. 9.4%; P<0.001) mortality and improved overall survival following sleeve resection. Sleeve resection was associated with 73% less mortality at 30 days and 57% less mortality at 90 days. Deslauriers et al. reported results of 1,230 consecutive patients with NSCLC who underwent sleeve resection (n=184) or pneumonectomy (n=1,046). Five-year overall survival was 52% following sleeve lobectomy and 31% after pneumonectomy (P<0.0001) (35). In a matching analysis performed by Park et al., postoperative mortality was substantially lower after sleeve procedures (1% vs. 8.6%), with greatly improved 5-year overall survival (58.4% vs. 32.1%) (44). Balduyck et al. demonstrated superior quality of life after sleeve lobectomy, with lower incidence of dyspnea, general pain, thoracic pain, and shoulder dysfunction (45). Collectively, if equivalent oncologic outcomes are anticipated, sleeve lobectomy is favored over pneumonectomy. The differences in morbidity, mortality, and survival after sleeve lobectomy versus pneumonectomy are highlighted in Table 2.
Table 2
| Study | PSM | No. of patients | Induction therapy (%) | Mortality (%) | Morbidity (%) | 5-year survival (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SL | PN | SL | PN | SL | PN | SL | PN | SL | PN | ||||||
| Okada (46) 2000 | Yes | 60 | 60 | NA | NA | 0 | 2 | 10 | 22 | 48 | 29 | ||||
| Lausberg (47) 2000 | No | 81 | 40 | NA | NA | 1.2 | 7.5 | NA | NA | 61.9 (2-year) | 56.1 (2-year) | ||||
| Martin-Ucar (48) 2002 | No | 38 | 81 | NA | NA | 10.5 | 9.9 | NA | NA | 64 (1-year) | 73 (1-year) | ||||
| Deslauriers (35) 2004 | No | 184 | 1046 | NA | NA | 1.3 | 5.3 | NA | NA | 52 | 31 | ||||
| Bagan (49) 2005* | No | 66 | 151 | 0 | 0 | 4.5 | 12.6 | 28.8 | 29.9 | 72.5 | 53.2# | ||||
| Kim (50) 2005 | No | 49 | 200 | 12 | 0 | 6.1 | 4.1 | 51 | 35 | 53.7 | 59.5 | ||||
| Ludwig (51) 2005 | No | 116 | 194 | NA | NA | 4.3 | 4.6 | 37.9 | 25.8 | 39.0 | 27.0 | ||||
| Takeda (52) 2006 | No | 62 | 110 | 25.8 | 16.8 | 4.8 | 3.6 | 45.2 | 40.9 | 54.3 | 32.9 | ||||
| Park (44) 2010 | Yes | 105 | 105 | 17.1 | 15.2 | 1.0 | 8.6 | 33.4 | 29.5 | 58.4 | 32.1 | ||||
| Gómez-Caro (53) 2011 | No | 55 | 21 | 20.0 | 9.5 | 3.6 | 4.7 | 32.3 | 33.3 | 61 | 31 | ||||
| Berry (39) 2014 | No | 35 | 52 | 0 | 0 | 5.7 | 3.9 | 49 | 58 | 65.2 (3-year) | 46.8 (3-year) | ||||
| Cusumano (54) 2014 | No | 51 | 68 | 100.0 | 100.0 | 3.9 | 2.9 | 47.0 | 48.6 | 53.8 | 43.1 | ||||
| Abdelsattar (43) 2017 | Yes | 1,713 | 1713 | 9.6 | 9.2 | 1.6 | 5.9 | NA | NA | NA | NA | ||||
| Pagès (55) 2017 | Yes | 794 | 794 | 20 | 21 | 4.99 | 5.89 | NA | NA | 71.86 (3-year) | 60.76 (3-year) | ||||
*Only right upper sleeve lobectomy vs. right pneumonectomy in this trial; #Right pneumonectomy with N0-1 disease or only skip metastasis. PSM, propensity score matching; SL, sleeve lobectomy; PN, pneumonectomy; NA, not available.
Nodal involvement
In the absence of lymph node involvement (N0), sleeve lobectomy is clearly superior to pneumonectomy. However, the use of sleeve lobectomy is slightly more controversial in cases of N1 involvement. This is because most patients with N1 disease die because of distant metastases rather than local recurrence (56). Therefore, N1 disease should not be considered a contraindication for a sleeve resection (56). Berry et al. concluded that, compared with pneumonectomy, sleeve lobectomy for NSCLC with N1 nodal disease does not compromise long-term survival (39).
Although several studies have reported an increased risk of recurrence after sleeve lobectomy, compared with pneumonectomy, for tumors with hilar adenopathy (50,56), other evidence suggests that the presence of N1 or N2 disease does not necessarily mandate pneumonectomy, and sleeve lobectomy can achieve complete tumor resection without a significantly increased risk of local recurrence or difference in survival (57-59). In fact, Okada et al. concluded that sleeve lobectomy should be performed, instead of pneumonectomy, regardless of nodal status (46).
The role of chemotherapy and radiation in pneumonectomy
The Intergroup trial (INT0139) performed in North America and reported in 2009 remains one of the most influential studies to evaluate surgery for stage III NSCLC (60). Patients received concurrent chemotherapy plus radiation to 45 Gy with or without surgical resection. Thirty percent of patients underwent pneumonectomy, with an observed 5-year overall survival of 22% and no improvement in long-term outcomes. Overall perioperative mortality rate was 26%, with 38% for right-side and 12% for left-side pneumonectomy, suggesting that pneumonectomy is a “high-risk” procedure in patients with stage III NSCLC who have received induction therapy (60). However, multiple, single-institution, retrospective studies have shown acceptable perioperative risk for pneumonectomy in the setting of induction chemotherapy and radiotherapy (61-65). In addition, when the literature is investigated for short-term mortality after pneumonectomy, there is no difference in 30-day mortality between patients who underwent neoadjuvant therapy (5.6%) and those who did not (6%) (66).
In 2012, Kim et al. published a meta-analysis that investigated 27 trials for perioperative mortality after pneumonectomy with induction chemotherapy and radiation (67). Mortality rates at 30 and 90 days were 7% and 12%, respectively, with no difference between patients whose induction regimen included radiation and those whose did not. The best prospective, contemporary data regarding safety of pneumonectomy following induction therapy comes from the SAKK trial, where 232 patients with resectable IIIA N2 disease were treated with either induction chemotherapy or chemotherapy and sequential radiation (68). Pneumonectomy was performed in 20% of patients in the trimodality group and 25% of patients in the bimodality group, with a low perioperative mortality of 4.5%. Again, no difference in survival was noted between the tri- and bimodality groups. A phase II trial published in 2009 reported a 5-year survival of 40% in patients who underwent induction chemoradiotherapy followed by surgery for stage IIIB disease (69). Pneumonectomy was performed in 48% of patients with a 30-day mortality rate of 5.7% and median overall survival of 29 months. Table 3 lists the study details and mortality and survival rates for phase III trials involving neoadjuvant therapy followed by pneumonectomy. Given these results, if oncologically indicated, the evidence strongly supports that a pneumonectomy can safely be performed following induction chemotherapy with or without radiation for locoregionally advanced NSCLC.
Table 3
| Study | Eligibility | Study arms | Preoperative RT | Dose (Gy) | No. of patients | Mortality (no. %) | 5-year survival (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Right | Left | All | Right | Left | |||||||
| Albain (60) 2009, Intergroup 0139 | T1-3pN2 | Induction RT-CHT + S vs. CHT + RT | Yes | 45 | 54 | 29 | 25 | 14 (26%) | 11 (38%) | 3 (12%) | 22 | |
| van Meerbeeck (70) 2007, EORTC 08941 | III/N2 (inoperable) | Induction CHT + S vs. CHT + RT | No | NA | 72 | 38 | 33 | 5 (7%) | 2 (5%) | 3 (9%) | NA | |
| Stephens (71) 2005 | T3N1, T1-3N2 (inoperable) | Induction CHT + S vs. RT | No | NA | 2 | NA | NA | 2 (100%) | NA | NA | NA | |
| Johnstone (72) 2002, RTOG 89-01 | T1-3pN2 | Induction CHT + S vs. CHT + RT | No | NA | NA | NA | NA | NA | NA | NA | NA | |
| Eberhardt (73) 2015, ESPATUE | IIIA (pN2), selected IIIB | Induction CHT + RT-CHT + S vs. Induction CHT + RT-CHT | Yes | 45 | 23 | NA | NA | 0 (0%) | NA | NA | NA | |
| Pless (68) 2015, SAKK | T1-3pN2 | Induction CHT + RT + S vs. Induction CHT + S | Some | 44 | 25 (CRS arm) | NA | NA | 0 (0%) | 0 (0%) | 0 (0%) | NA | |
| 19 (CS arm) | NA | NA | 3 (16%) | 2 (NA) | 1 (NA) | NA | ||||||
| Katakami (74) 2012, WJTOG9903 | T1-3pN2 | Induction CHT + RT + S vs. Induction CHT + S | Some | 40 | 0 (CRS arm) | 0 | 0 | NA | NA | NA | NA | |
| 1 (CS arm) | NA | NA | 0 (0%) | NA | NA | NA | ||||||
| Thomas (75) 2008, GLCCG | T1-3N2 or central T3N0-1, T4N1-3 or T1-4N3 | Induction CHT + RT-CHT + S vs. Induction CHT + S + PORT | Some | 45 | 50 (Inter arm) | 19 | 31 | 7 (14%) | NA | NA | NA | |
| 54 (Control arm) | 20 | 34 | 3 (6%) | NA | NA | NA | ||||||
CHT, chemotherapy; S, surgery; RT, radiotherapy; NA, not available; CRS arm, Induction CHT + RT + S arm; CS arm, Induction CHT + S arm; PORT, postoperative radiotherapy; Inter arm, Induction CHT + RT-CHT + S arm; Control arm, Induction CHT + S + PORT arm.
Operative technique
A standard muscle-sparing posterolateral thoracotomy in the fifth or occasionally fourth intercostal space provides optimal exposure. The latissimus dorsi muscle is divided, and the serratus anterior muscle is retracted superiorly. The rib can be transected posteriorly to facilitate exposure. Preservation of the intercostal muscle should be done at this time in case it is needed to cover the bronchial stump. On entering the pleural space, absence of pleural effusion and intrathoracic metastatic spread should be confirmed. Thoracoscopic evaluation can be used for this purpose as well. Thoracoscopy helps to identify pleural metastases that may not be identified by conventional imaging techniques and can also be used for surgical planning to determine the optimal incision used for resection (76).
Left-sided pneumonectomy
We initially perform a complete mediastinal lymph node dissection including stations 5, 6, 7, 9, and, if enlarged or FDG-avid, 4L and 8. Particular attention should be paid to the recurrent laryngeal and phrenic nerves to avoid injury during the dissection. The pulmonary veins and main pulmonary artery (PA) are then isolated and divided using the stapler. Safe dissection of these structures is always facilitated by first removing the hilar N1 nodes whenever possible. With central tumors, the hilar dissection can be challenging, and it may require opening the pericardium. Given the short intrapericardial length of the left pulmonary artery, identification and division of the ligamentum arteriosum may be performed to maximize length on the main PA.
The left mainstem bronchus is circumferentially dissected, with care being taken not to devascularize the airway. Again, a complete station 7 nodal dissection should be performed, as this makes the subsequent bronchial dissection relatively easy. The endobronchial extent of the tumor determined by preoperative bronchoscopy is taken into consideration before division of the bronchus. Bronchial margins are then sent for frozen section analysis. The bronchial stump should be <2 cm and is then tested for pneumostasis by submerging it under water. If no leak is identified, the bronchial stump may be bolstered with soft tissue (intercostal muscle, pericardial fat pad, or pedicled pericardium) to help prevent bronchopleural fistula. For left-sided pneumonectomies, we do not routinely cover the bronchus as it is covered in large part by the arch of the aorta. However, if induction radiation was administered or adjuvant radiation is being considered, bronchial stump coverage may be performed.
Right-sided pneumonectomy
Surgical principles and approach are similar to those previously described for left-sided pneumonectomy. Following exposure, we perform a complete mediastinal lymph node dissection including stations 2R, 4R, 7, 9, and occasionally level 8 nodes. Assessment of the hilum and vascular control is performed next. The azygous vein is not routinely divided, but this can be performed to facilitate exposure to the proximal right main PA and the mainstem bronchus. The superior vena cava is mobilized to gain control of the main PA. If that proves challenging secondary to tumor location or treatment effect, one should open the pericardium and obtain control medial to the superior vena cava. If significant resection of the pericardium is required to achieve an R0 resection, the pericardium should be reconstructed with prosthetic material such as Gore-Tex mesh or polypropylene mesh. This is done to prevent cardiac herniation—a potentially highly lethal complication (77,78).
Postoperative management, complications, and outcomes
Initial management of patients following pneumonectomy consists of early extubation (preferably in the operating room), adequate pain relief (usually with an epidural catheter), aggressive pulmonary toilet, early ambulation, and venous thromboprophylaxis (79). Despite this, 33% to 44% of patients experience a complication after pneumonectomy (80-82). One of the most common complications is atrial dysrhythmia, occurring in approximately 20% to 40% of patients following pneumonectomy (81,83). More severe complications after pneumonectomy, such as empyema, acute respiratory distress syndrome (ARDS), and bronchopleural fistula (BPF), are less common but are independent risk factors for death. A recent report of the Memorial Sloan Kettering (MSK) experience with pneumonectomy considered 355 patients over an 18-year period who underwent pneumonectomy for NSCLC. In this series, an incidence of BPF of 3.7% was found (84), which is similar to rates (8%) in other studies (82,85). Postpneumonectomy ARDS occurred in 2.3% of cases (84), compared with rates of 2.7% to 12% in other studies (81,82,86,87). Postpneumonectomy ARDS is associated with postoperative mortality of almost 70% and 5-year overall survival of 17.6% (88). Primary risk factors for developing BPF and ARDS are older age and right-sided pneumonectomy (81,82,85). Right-sided pneumonectomy is associated with a higher risk of death, compared with left-sided pneumonectomy, especially after neoadjuvant therapy (89). Although induction chemoradiation was found to be an independent risk factor for major adverse events following pneumonectomy in a large study from the Society of Thoracic Surgeons General Thoracic Surgery Database (90), other studies have not supported this association (82,85). Mortality rates after pneumonectomy also appear to be influenced by hospital volume. The unadjusted in-hospital mortality for 90,088 patients undergoing pneumonectomy in the Healthcare Cost and Utilization Project Nationwide Inpatient Sample database was 2.7% in the highest-volume decile of hospitals versus 4.9% in the lowest-volume decile (91). As a comparison, in the MSK series, the reported 30-day mortality was 4.6% (84). Moreover, the mortality burden after pneumonectomy should not be overlooked in the post-hospital discharge setting. Schneider et al. reported that more than half of postpneumonectomy deaths occurred during the first 90 days after discharge from the hospital (92). In the MSK series, 13 of 24 deaths within the first 90 days occurred while in-hospital (84).
In general, factors associated with adverse long-term survival include increased age, advanced stage, extended resection, adenocarcinoma, lymphatic vessel microinvasion, lymph node involvement, and residual disease (93). Conversely, sex, pneumonectomy laterality, and tumor size do not predict long-term survival (93,94). Fernandez et al. observed that, although right-sided pneumonectomy was associated with an approximately 2-fold increase in perioperative mortality, compared with left-sided pneumonectomy, 3-year survival rates were similar (39% for right vs. 41% for left) (92).
Conclusions
Pneumonectomy is an increasingly less common surgical procedure and is often the last resort for patients with NSCLC not amenable to lung-sparing resection. Sleeve resection has shown promise as an alternative to pneumonectomy, although in cases of centrally located tumors, involvement of vascular structures, or bulky hilar adenopathy, pneumonectomy may be unavoidable. In these cases, pneumonectomy can be performed with acceptable operative morbidity and mortality, even after induction therapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lorenzo Spaggiari and Luca Bertolaccini) for the series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.12.05). The series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” was commissioned by the editorial office without any funding or sponsorship. GR serves as the Editor-in-Chief of Shanghai Chest and reports other from Scanlan, outside the submitted work. DRJ reports other from Diffusion Pharmaceuticals, other from AstraZeneca, and other from Merck, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cronin KA, Lake AJ, Scott S, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018;124:2785-800. [Crossref] [PubMed]
- Park S, Park IK, Kim ER, et al. Current trends of lung cancer surgery and demographic and social factors related to changes in the trends of lung cancer surgery: an analysis of the national database from 2010 to 2014. Cancer Res Treat 2017;49:330-7. [Crossref] [PubMed]
- Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016;893:1-19. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313.
- Le Chevalier T. Adjuvant chemotherapy for resectable non-small cell lung cancer: where is it going? Ann Oncol 2010;21:vii196-8. [Crossref] [PubMed]
- Siripurapu V, Stitzenberg KB, Nitzkorski J, et al. Pneumonectomy for cancer in the Mid-Atlantic US: trends and outcomes over a decade. Chest 2010;138:659A. [Crossref]
- Rocco G, Nason K, Brunelli A, et al. Management of stage IIIA (N2) non-small-cell lung cancer: a transatlantic perspective. Eur J Cardiothorac Surg 2016;49:1025. [Crossref] [PubMed]
- Wolf AS, Daniel J, Sugarbaker D. Surgical techniques for multimodality treatment of malignant pleural mesothelioma: extrapleural pneumonectomy and pleurectomy/decortication. Semin Thorac Cardiovasc Surg 2009;21:132-48. [Crossref] [PubMed]
- Yalman D. Neoadjuvant radiotherapy/chemoradiotherapy in locally advanced non-small cell lung cancer. Balkan Med J 2015;32:1-7. [Crossref] [PubMed]
- Allen AM, Mentzer SJ, Yeap BY, et al. Pneumonectomy after chemoradiation. Cancer 2008;112:1106-13. [Crossref] [PubMed]
- Bott MJ, Patel AP, Crabtree TD, et al. Role for surgical resection in the multidisciplinary treatment of stage IIIB non-small cell lung cancer. Ann Thorac Surg 2015;99:1921-8. [Crossref] [PubMed]
- Alexiou C, Beggs D, Onyeaka P, et al. Pneumonectomy for stage I (T1N0 and T2N0) nonsmall cell lung cancer has potent, adverse impact on survival. Ann Thorac Surg 2003;76:1023-8. [Crossref] [PubMed]
- Shah AA, Worni M, Kelsey CR, et al. Does pneumonectomy have a role in the treatment of stage IIIA non-small cell lung cancer? Ann Thorac Surg 2013;95:1700-7. [Crossref] [PubMed]
- Simón C, Moreno N, Peñalver R, et al. The side of pneumonectomy influences long-term survival in stage I and II non-small cell lung cancer. Ann Thorac Surg 2007;84:952-8. [Crossref] [PubMed]
- Deslauriers D, Ugalde P, Miro S, et al. Long-term physiological consequences of pneumonectomy. Semin Thorac Cardiovasc Surg 2011;23:196-202. [Crossref] [PubMed]
- Ramnath N, Demmy TL, Antun A, et al. Pneumonectomy for bronchogenic carcinoma: analysis of factors predicting survival. Ann Thorac Surg 2007;83:1831-6. [Crossref] [PubMed]
- Nissen R, Wilson RHL. Pages in the history of chest surgery. Springfield, IL: Charles C. Thomas; 1960.
- Haight C. Total removal of the left lung for bronchiectasis. Surg Gynecol Obstet 1934;58:768.
- Graham EA. The first pneumonectomy. Tex Cancer Bull 1949;2:2. [PubMed]
- Overholt R. Pneumonectomy for malignant and suppurative disease of the lung. J Thorac Cardiovasc Surg 1939;9:17.
- Crafoord C. On the technique of pneumonectomy in man. Acta Chir Scand 1938;81:54.
- Walker WS, Carnochan F, Mattar S. Video-assisted thoracoscopic pneumonectomy. Br J Surg 1994;81:81-2. [Crossref] [PubMed]
- Conlan AA, Sandor A. Total thoracoscopic pneumonectomy: indications and technical considerations. J Thorac Cardiovasc Surg 2003;126:2083-5. [Crossref] [PubMed]
- Nwogu CE, Glinianski M, Demmy TL. Minimally invasive pneumonectomy. Ann Thorac Surg 2006;82:e3-4. [Crossref] [PubMed]
- Spaggiari L, Galetta D. Pneumonectomy for lung cancer: a further step in minimally invasive surgery. Ann Thorac Surg 2011;91:e45-7. [Crossref] [PubMed]
- American Thoracic Society. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211-77. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Boysen PG, Harris JO, Block AJ, et al. Prospective evaluation for pneumonectomy using perfusion scanning: follow-up beyond one year. Chest 1981;80:163-6. [Crossref] [PubMed]
- Zhu X, Zhao M, Liu C, et al. Prediction of the postoperative pulmonary function in lung cancer patients with borderline function using ventilation-perfusion scintigraphy. Nucl Med Commun 2012;33:283-7. [Crossref] [PubMed]
- Beckles MA, Spiro SG, Colice GL, et al. The physiologic evaluation of patients with lung cancer being considered for resectional surgery. Chest 2003;123:105S-14S. [Crossref] [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [Crossref] [PubMed]
- Villani F, Busia A. Preoperative evaluation of patients submitted to pneumonectomy for lung carcinoma: role of exercise testing. Tumori 2004;90:405-9. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: lung cancer screening. Version I. 2017. Available online: www.nccn.org. Accessed December 1, 2019.
- Deslauriers J, Grégoire J, Jacques LF, et al. Sleeve lobectomy versus pneumonectomy for lung cancer: a comparative analysis of survival and sites or recurrences. Ann Thorac Surg 2004;77:1152-6; discussion 1156. [Crossref] [PubMed]
- Thomas CP. Conservative resection of the bronchial tree. J R Coll Surg Edinb 1956;1:169-86. [PubMed]
- Paulson DL, Shaw RR. Results of bronchoplastic procedures for bronchogenic carcinoma. Ann Surg 1960;151:729-40. [Crossref] [PubMed]
- Perentes JY, Zellweger M, Gonzalez M. Is pneumonectomy still necessary? J Thorac Dis 2018;10:6414-7. [Crossref] [PubMed]
- Berry MF, Worni M, Wang X, et al. Sleeve lobectomy for non-small cell lung cancer with N1 nodal disease does not compromise survival. Ann Thorac Surg 2014;97:230-5. [Crossref] [PubMed]
- Ma QL, Guo YQ, Shi B, et al. For non-small cell lung cancer with T3 (central) disease, sleeve lobectomy or pneumonectomy? J Thorac Dis 2016;8:1227-33. [Crossref] [PubMed]
- Maurizi G, D’Andrilli A, Anile M, et al. Sleeve lobectomy compared with pneumonectomy after induction therapy for non-small-cell lung cancer. J Thorac Oncol 2013;8:637-43. [Crossref] [PubMed]
- Maurizi G, Ciccone AM, Vanni C, et al. Reimplantation of the upper lobe bronchus after lower sleeve lobectomy or bilobectomy: long-term results. Eur J Cardiothorac Surg 2018;53:1180-5. [Crossref] [PubMed]
- Abdelsattar ZM, Shen KR, Yendamuri S, et al. Outcomes after sleeve lung resections versus pneumonectomy in the United States. Ann Thorac Surg 2017;104:1656-64. [Crossref] [PubMed]
- Park JS, Yang HC, Kim HK, et al. Sleeve lobectomy as an alternative procedure to pneumonectomy for non-small cell lung cancer. J Thorac Oncol 2010;5:517-20. [Crossref] [PubMed]
- Balduyck B, Hendriks J, Lauwers P, et al. Quality of life after lung cancer surgery: a prospective pilot study comparing bronchial sleeve lobectomy with pneumonectomy. J Thorac Oncol 2008;3:604-8. [Crossref] [PubMed]
- Okada M, Yamagishi H, Satake S, et al. Survival related to lymph node involvement in lung cancer after sleeve lobectomy compared with pneumonectomy. J Thorac Cardiovasc Surg 2000;119:814-9. [Crossref] [PubMed]
- Lausberg HF, Graeter TP, Wendler O, et al. Bronchial and bronchovascular sleeve resection for treatment of central lung tumors. Ann Thorac Surg 2000;70:367-71; discussion 371-2. [Crossref] [PubMed]
- Martin-Ucar AE, Chaudhuri N, Edwards JG, et al. Can pneumonectomy for non-small cell lung cancer be avoided? An audit of parenchymal sparing lung surgery. Eur J Cardiothorac Surg 2002;21:601-5. [Crossref] [PubMed]
- Bagan P, Berna P, Pereira JC, et al. Sleeve lobectomy versus pneumonectomy: tumor characteristics and comparative analysis of feasibility and results. Ann Thorac Surg 2005;80:2046-50. [Crossref] [PubMed]
- Kim YT, Kang CH, Sung SW, et al. Local control of disease related to lymph node involvement in non-small cell lung cancer after sleeve lobectomy compared with pneumonectomy. Ann Thorac Surg 2005;79:1153-61; discussion 1161. [Crossref] [PubMed]
- Ludwig C, Stoelben E, Olschewski M, et al. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg 2005;79:968-73. [Crossref] [PubMed]
- Takeda S, Maeda H, Koma M, et al. Comparison of surgical results after pneumonectomy and sleeve lobectomy for non-small cell lung cancer: trends over time and 20-year institutional experience. Eur J Cardiothorac Surg 2006;29:276-80. [Crossref] [PubMed]
- Gómez-Caro A, Garcia S, Reguart N, et al. Determining the appropriate sleeve lobectomy versus pneumonectomy ratio in central non-small cell lung cancer patients: an audit of an aggressive policy of pneumonectomy avoidance. Eur J Cardiothorac Surg 2011;39:352-9. [Crossref] [PubMed]
- Cusumano G, Marra A, Lococo F, et al. Is sleeve lobectomy comparable in terms of short- and long-term results with pneumonectomy after induction therapy? A multicenter analysis. Ann Thorac Surg 2014;98:975-83. [Crossref] [PubMed]
- Pagès PB, Mordant P, Renaud S, et al. Sleeve lobectomy may provide better outcomes than pneumonectomy for non-small cell lung cancer. A decade in a nationwide study. J Thorac Cardiovasc Surg 2017;153:184-95.e3. [Crossref] [PubMed]
- Erino AR, Venuta F, De Giacomo T, et al. Sleeve resection after induction therapy. Thorac Surg Clin 2004;14:191-7. [Crossref] [PubMed]
- Ferguson MK, Lehman AG. Sleeve lobectomy or pneumonectomy: optimal management strategy using decision analysis techniques. Ann Thorac Surg 2003;76:1782-8. [Crossref] [PubMed]
- Merritt RE, Mathisen DJ, Wain JC, et al. Long-term results of sleeve lobectomy in the management of non-small cell lung carcinoma and low-grade neoplasms. Ann Thorac Surg 2009;88:1574-81. [Crossref] [PubMed]
- Rendina EA, Venuta F, de Giacomo T, et al. Parenchymal sparing operations for bronchogenic carcinoma. Surg Clin North Am 2002;82:589-609. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Decaluwé H, De Leyn P, Vansteenkiste J, et al. Surgical multimodality treatment for baseline resectable stage IIIA-N2 non-small cell lung cancer. Degree of mediastinal lymph node involvement and impact on survival. Eur J Cardiothorac Surg 2009;36:433-9. [Crossref] [PubMed]
- Ferguson MK, Karrison T. Does pneumonectomy for lung cancer adversely influence long-term survival? J Thorac Cardiovasc Surg 2000;119:440-8. [Crossref] [PubMed]
- Friedel G, Budach W, Dippon J, et al. Phase II trial of a trimodality regimen for stage III non-small-cell lung cancer using chemotherapy as induction treatment with concurrent hyperfractionated chemoradiation with carboplatin and paclitaxel followed by subsequent resection: a single-center study. J Clin Oncol 2010;28:942-8. [Crossref] [PubMed]
- Stamatis G, Eberhard W, Pottgen C. Surgery after multimodality treatment for non-small-cell lung cancer. Lung Cancer 2004;45:S107-12. [Crossref] [PubMed]
- Paul S, Mirza F, Port JL, et al. Survival of patients with clinical stage IIIA non-small cell lung cancer after induction therapy: age, mediastinal downstaging, and extent of pulmonary resection as independent predictors. J Thorac Cardiovasc Surg 2011;141:48-58. [Crossref] [PubMed]
- Alan S, Jan S, Tomas H, et al. Does chemotherapy increase morbidity and mortality after pneumonectomy? J Surg Oncol 2009;99:38-41. [Crossref] [PubMed]
- Kim AW, Boffa DJ, Wang Z, et al. An analysis, systematic review, and meta-analysis of the perioperative mortality after neoadjuvant therapy and pneumonectomy for non-small cell lung cancer. J Thorac Cardiovasc Surg 2012;143:55-63. [Crossref] [PubMed]
- Pless M, Stupp R, Ris H-B, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015;386:1049-56. [Crossref] [PubMed]
- Stupp R, Mayer M, Kann R, et al. Neoadjuvant chemotherapy and radiotherapy followed by surgery in selected patients with stage IIIB non-small-cell lung cancer: a multicentre phase II trial. Lancet Oncol 2009;10:785-93. [Crossref] [PubMed]
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442-50. [Crossref] [PubMed]
- Stephens RJ, Girling DJ, Hopwood P, et al. Medical Research Council Lung Cancer Working P. A randomized controlled trial of pre-operative chemotherapy followed, if feasible, by resection versus radiotherapy in patients with inoperable stage T3, N1, M0 or T1-3, N2, M0 non-small cell lung cancer. Lung Cancer 2005;49:395-400. [Crossref] [PubMed]
- Johnstone DW, Byhardt RW, Ettinger D, et al. Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 2002;54:365-9. [Crossref] [PubMed]
- Eberhardt WE, Pottgen C, Gauler TC, et al. Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non-small-cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE). J Clin Oncol 2015;33:4194-201. [Crossref] [PubMed]
- Katakami N, Tada H, Mitsudomi T, et al. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer 2012;118:6126-35. [Crossref] [PubMed]
- Thomas M, Rube C, Hoffknecht P, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol 2008;9:636-48. [Crossref] [PubMed]
- Roberts JR, Blum MG, Arildsen R, et al. Prospective comparison of radiologic, thoracoscopic, and pathologic staging in patients with early non-small cell lung cancer. Ann Thorac Surg 1999;68:1154-8. [Crossref] [PubMed]
- Kawamukai K, Antonacci F, Di Saverio S, et al. Acute postoperative cardiac herniation. Interact Cardiovasc Thorac Surg 2011;12:73-4. [Crossref] [PubMed]
- Mehanna MJ, Israel GM, Katigbak M, et al. Cardiac herniation after right pneumonectomy: case report and review of the literature. J Thorac Imaging 2007;22:280-2. [Crossref] [PubMed]
- Shargall Y, Brunelli A, Murthy S, et al. Venous thromboembolism prophylaxis in thoracic surgery patients: an international survey. Eur J Cardiothorac Surg 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Saha SP, Kalathiya RJ, Davenport DL, et al. Survival after pneumonectomy for stage III non-small cell lung cancer. Oman Med J 2014;29:24-7. [Crossref] [PubMed]
- Thomas PA, Berbis J, Baste JM, et al. Pneumonectomy for lung cancer: contemporary national early morbidity and mortality outcomes. J Thorac Cardiovasc Surg 2015;149:73-82. [Crossref] [PubMed]
- Darling GE, Abdurahman A, Yi QL, et al. Risk of a right pneumonectomy: role of bronchopleural fistula. Ann Thorac Surg 2005;79:433-7. [Crossref] [PubMed]
- Onaitis M, D'Amico T, Zhao Y, et al. Risk factors for atrial fibrillation after lung cancer surgery: analysis of the Society of Thoracic Surgeons general thoracic surgery database. Ann Thorac Surg 2010;90:368-74. [Crossref] [PubMed]
- Jones GD, Caso R, Tan KS, et al. What really happens at 30 and 90 days after pneumonectomy? J Thorac Cardiovasc Surg 2019; In press.
- Deschamps C, Bernard A, Nichols FC III, et al. Empyema and bronchopleural fistula after pneumonectomy: factors affecting incidence. Ann Thorac Surg 2001;72:243-7; discussion 248. [Crossref] [PubMed]
- Kutlu CA, Williams EA, Evans TW, et al. Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Ann Thorac Surg 2000;69:376-80. [Crossref] [PubMed]
- Jeon K, Yoon JW, Suh GY, et al. Risk factors for post-pneumonectomy acute lung injury/acute respiratory distress syndrome in primary lung cancer patients. Anaesth Intensive Care 2009;37:14-9. [Crossref] [PubMed]
- Blanc K, Zaimi R, Dechartres A, et al. Early acute respiratory distress syndrome after pneumonectomy: presentation, management, and short-and long-term outcomes. J Thorac Cardiovasc Surg 2018;156:1706-14.e5. [Crossref] [PubMed]
- Martin J, Ginsberg RJ, Abolhoda A, et al. Morbidity and mortality after neoadjuvant therapy for lung cancer: the risks of right pneumonectomy. Ann Thorac Surg 2001;72:1149-54. [Crossref] [PubMed]
- Shapiro M, Swanson SJ, Wright CD, et al. Predictors of major morbidity and mortality after pneumonectomy utilizing the Society for Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg 2010;90:927-34; discussion 934-5. [Crossref] [PubMed]
- Hollenbeck BK, Dunn RL, Miller DC, et al. Volume-based referral for cancer surgery: informing the debate. J Clin Oncol 2007;25:91-6. [Crossref] [PubMed]
- Schneider L, Farrokhyar F, Schieman C, et al. Pneumonectomy: the burden of death after discharge and predictors of surgical mortality. Ann Thorac Surg 2014;98:1976-81. [Crossref] [PubMed]
- Riquet M, Mordant P, Pricopi C, et al. A review of 250 ten-year survivors after pneumonectomy for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:876-81. [Crossref] [PubMed]
- Doddoli C, Barlesi F, Trousse D, et al. One hundred consecutive pneumonectomies after induction therapy for nonsmall cell lung cancer: an uncertain balance between risks and benefits. J Thorac Cardiovasc Surg 2005;130:416-25. [Crossref] [PubMed]
Cite this article as: Yan S, Gritsiuta AI, Medrano del Rosal G, Jones G, Rocco G, Jones DR. Pneumonectomy for lung cancer. Shanghai Chest 2020;4:25.