Management of malignant central airway obstruction
Clinical background
Central airway obstructions are generally defined as luminal obstructions of more than 50% in the trachea, mainstem bronchi, bronchus intermedius, or a lobar bronchus (1,2). Further, not only can these obstructions be categorized by the location in the central airway, but also by the type of tumor seen. Therefore, malignant central airway obstructions (MCAO) are further classified into intraluminal tumor growth (intrinsic), extraluminal tumor compression (extrinsic), or a combination of both (mixed) (3).
Locally advanced primary lung cancer is the most common etiology of MCAO (4). Of the roughly 200,000 new cases of primary lung cancer per year in the United States (5), an estimated 30% will develop clinically evident endoluminal disease (5-7). Other primary malignancies of the lung or airway that lead to MCAO include adenoid cystic carcinoma, mucoepidermoid carcinoma, and carcinoid tumors (8-10). Structures adjacent to the lungs including esophageal, thyroid, and primary mediastinal tumors have also been described as causes of MCAO (11). Although locally advanced lung cancer remains the most likely etiology of MCAO, airway metastases from virtually any malignancy have been reported as well, particularly thyroid, breast, colon, renal cancer, and melanoma (11).
Presentation
The aggressiveness and location of the tumor will cause variation in patient presentation. Expiratory wheezing suggests intrathoracic airway obstruction, often distal to the carina. Stridor is generally a sign of extrathoracic obstruction (8). Mild symptoms of MCAO include cough, wheezing and exertional dyspnea (12). These symptoms may often be mistaken for obstructive airways disease such as asthma or chronic obstructive pulmonary disease (COPD) and may lead to a delay in diagnosis.
Most often, MCAO presents with clear signs and symptoms. In addition to wheeze or stridor, patients may have resting dyspnea, hemoptysis, or a history of post obstructive infections (13,14). However, CAO can be asymptomatic and occasionally found incidentally on chest imaging (8,15,16).
Non-bronchoscopic management techniques
Non-bronchoscopic management of MCAO includes chemotherapy, external radiation, and surgical resection. Both chemotherapy and radiation are frequently employed to manage central airway obstructions, but have limitations in the acute setting. Both modalities attempt to achieve the goal of tumor size reduction, but also require an ample amount of time to observe any symptom relief, if at all. Therefore, these modalities alone are often not feasible due to the severe progression of patients’ symptoms and most likely obstruction during inspection of the airway. Furthermore, chemo- and radio-therapy alone seldom can alleviate airway obstruction due to non-small cell lung cancer (NSCLC) (17).
Surgery may play a role in curative intent, although most malignant airway lesions are surgically inoperable due to advanced disease stage and therefore, require multimodality palliation (18).
Introduction to bronchoscopic techniques
Patients with MCAO may present with various symptoms that require emergent (e.g., in cases of hemorrhage or acute respiratory failure) or non-emergent (e.g., ongoing or worsening dyspnea) intervention. Bronchoscopic techniques offer an ability to allow restoration of airway patency and improve palliative outcomes, regardless of the level of urgency (19). These options are often safer, cost effective, and pose less risk than benefit compared to invasive surgical options (20).
In patients that may tentatively undergo surgical intervention, these techniques may also provide great benefit through providing better visualization of the airways, resolution of atelectasis, and subsequent ability to treat post obstruction infections, eventually optimizing candidacy and decreasing risk of failure (21,22).
Direct mechanical modalities
Bronchoscopy
Both rigid and flexible bronchoscopy are able to provide diagnostic and therapeutic interventions. The preference of scope may depend on many factors such as better control of the airway or larger size of working channel. These scopes are often combined during a procedure with flexible bronchoscopy performed through the rigid barrel to allow distal airway access or more nuanced articulation.
Rigid bronchoscopy has historically served as an important mechanical modality for dealing with MCAO. The use of rigid bronchoscopy has especially been useful in patients with an acute, unstable, airway (8). We often find that this tool serves to provide immediate relief and stabilization through the ability of securing the airway. A clear advantage of its use is the large channel allowing for entry of different therapeutic tools, ease of airway stenting, suctioning through the tube, all while providing ventilation throughout the procedure (23). The bevel of the bronchoscope itself can be used for debulking or “coring out” of an obstruction (24). Furthermore, the barrel of the bronchoscope in itself can be utilized to tamponade bleeding central lesions seen in malignant obstructions (25).
Cryorecanalization
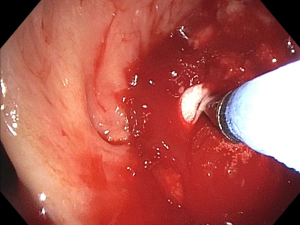
While the cryoprobe may be used for standard probe cryotherapy with freeze-thaw cycles that serves as an indirect or delayed therapy, it may also be utilized for cryorecanalization (26). Contrary to the mechanism of freeze-thaw cycles used in standard cryotherapy, cryorecanalization involves application of the cryoprobe directly applied to the tumor continuously for approximately 3–20 seconds, allowing freezing and adherence (26-30) (Figure 1). Quick removal of the cryoprobe then removes any frozen tissue still adherent to the probe (31). Cryorecanalization allows for retrieval of the frozen tumor for pathology while simultaneously debulking the obstruction with rapid withdrawal of the cryoprobe (26). The main advantage of this approach is immediate debulking of the tumor, with a lower risk of perforation or residual stenosis (26,29). The maneuver is most often done with flexible cryoprobes via flexible bronchoscopy.
The main disadvantage is removal of the specimen en bloc with the bronchoscope as the frozen tumor is too large to pull through the working channel of the flexible bronchoscope. This leads to temporary loss of airway visualization and limited ability to inspect for hemorrhage and stop bleeding until the specimen is thawed in saline and the bronchoscope is re-introduced (28). This limitation can be mitigated with the use of suction via a rigid bronchoscope or alternating with a second bronchoscope, when available. Serious bleeding can occur using this technique and should be used with caution at centers without experience managing pulmonary hemorrhage.
Microdebrider
This instrument is passed under direct telescopic guidance through rigid a bronchoscope or laryngoscope into the airway (3). Airway debulking is purely mechanical and performed by a rotating blade at the distal tip of the instrument (8). No pathologic specimen is obtained during the debulking process. The benefits of the microdebrider are the ability to rapidly and efficiently debulk while maintaining direct visualization through suctioning (8,27,32,33). Contrary to thermal modalities, the device does not pose a risk of airway fire and thus does not require a fraction of inspired oxygen (FiO2) less than 40% (24,32). As the microdebrider is a relatively new tool for debulking, further studies are required to evaluate safety and long-term outcomes.
Stents
Airway stenting for MCAO is indicated in patients with pure extrinsic compression or a mixed obstruction where there is still significant obstruction after endoluminal debulking (34). Metallic and silicone are the two main types of airway stents currently available (35). Compared to silicone stents, metallic stents have better internal-external diameter ratio and therefore lead to larger airway lumens, are radio-opaque, making them easy to spot in imaging, and lower incidence of migration (36,37). However, silicone stent offer more choices in shape and size, including the ability to stent multiple airways with a single stent.
Metallic stents can be divided into covered and uncovered, as well as self-expandable metallic stents (SEMS) or fixed-diameter stents requiring balloon dilation. Although uncovered stents in theory do not interrupt the mucociliary clearance, they can be difficult to remove due to tumor or granulation tissue overgrowth through the stent fenestrations. SEMS can be placed using prepackaged deployment catheters through existing airways such as by laryngeal mask airway or rigid bronchoscope with real time guidance using a small flexible bronchoscope or by fluoroscopy (36,38-45). Smaller stent deployment catheters can now fit through the 2.8 mm working channel of a therapeutic bronchoscope, allowing for easy direct visualization of stent deployment.
The main pitfalls of all stents are due to the rate of complications. Common complications include granulation tissue at the stent edges, epithelialization with incorporation into the mucosa, obstruction of the stent with mucous, stent migration with potential airway obstruction, airway injury with hemoptysis or perforation (37,46-50). Complications from stenting, including stent migration and stent obstruction by granulation tissue or secretions vary from approximately 20% to 50% (23,49). Regardless of the rates of complications, the overall mortality from stent placement is very low (38,51).
Direct thermal therapies
Similar to mechanical interventions, thermal techniques can provide immediate therapeutic relief through direct tissue destruction and relief of stenosis. Laser therapy, contact electrocautery, argon plasma coagulation (APC), and Corecath (Medtronic) have all been noted to be viable options for direct thermal intervention.
Laser therapy
Light amplification of stimulated emission of radiation (LASER), uses its properties to deliver a precise beam of thermal energy through a thin fiber to coagulate and vaporize tissue (4,52-54). The coagulative properties of laser can be helpful in providing hemostasis with superficial hemorrhagic lesions while the ablative properties of the laser are beneficial in debulking endoluminal masses (24). Flexible laser fibers can be positioned within the airway through the use of a flexible or rigid bronchoscope.
Tissue coagulation can be provided by a shallow laser effect at low power settings whereas high power settings allow for more penetration with more resultant carbonization and vaporization (24). Position of the laser fiber further (1 cm or greater) cause superficial penetration and better coagulation whereas closer distances (3–4 mm) cause deeper penetration and more efficient vaporization (24,52).
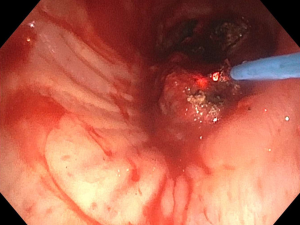
Several large studies have demonstrated the utility and effectiveness of neodymium-yttrium-aluminum-garnet (Nd:YAG) laser photoradiation in airway tumor debulking. The technique involves a flexible fiber inserted through either rigid or flexible bronchoscope to emit the light beam (4). As the tissue penetration of Nd:YAG laser is approximately 10 mm, comparatively deeper than electrocautery and APC, the laser is directed parallel to the airway wall to reduce perforation and bronchovascular fistula (4,7,52) (Figure 2). Drawbacks included the inability to use this modality with higher oxygen requirements (FiO2 >40%), similar to other thermal ablative techniques (55).
The use of Nd:YAG laser in the treatment of MCAO provides immediate palliation of symptoms (24,56-59), while allowing for minimal bleeding during debulking (55). If done with proper precautions, NDYAG has an excellent safety record (57,60-66).
Electrocautery
The utility of electrocautery devices is their simplicity, rapid palliation, and immediate tumor debulking (67). Through the use of a probe or device targeting tissue, electrocautery permits high-frequency electrical currents to be converted to heat energy for tissue coagulation or dissection of tumor tissue (68). The effects of thermal heat delivered through the use of electrocautery are dependent on the power and voltage of the electrocautery, tissue resistance, temperature at the tissue, and duration (52). Those factors will delineate if coagulation, hemostasis, carbonization, or vaporization will occur (69).
Electrocautery can be performed with either flexible bronchoscope or rigid bronchoscopy (55). Soft or forced coagulation is used at 20 to 40 Watts in short bursts of >5 seconds with the blunt probe (70). This facilitates coagulation, at which point tissue can be removed through mechanical debridement. Increasing the activation and therefore contact time may also lead to tissue carbonization and vaporization (52). For lesions resting on a “stalk”, a snare may be utilized around the stalk to cut and subsequently coagulate the stalk, remove the tumor en mass. An electrocautery knife allows for more precise debulking of tumor (69). Electrocautery forceps allow for simultaneous tissue biopsy and subsequent cauterization though care must be taken to minimize thermal damage to sampled tissues (4,71).
Potential complications include hemorrhage, airway perforation, airway fire, and scarring/stenosis (72). Care should be taken maintain an FiO2 level of less than 40%, and a power setting of less than 80 W, and application times greater than 5 seconds (54,69).
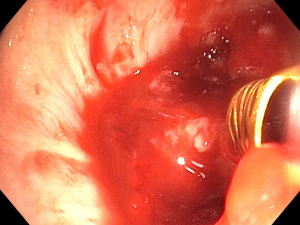
The Corecath (Medtronic) is a new electrocautery device that can be used with a flexible bronchoscope for therapeutic debulking of MCAOs. Through the use of flexible bronchoscopy, the Corecath allows for an electrosurgical means to debulk obstructions, provide hemostasis, and surgical smoke and blood evacuation through its integrated suction (73) (Figure 3).
Overall, electrocautery is a cost friendly, efficacious, and safe modality in management of MCAO (20,74-76), with a success rate comparable to Nd:YAG laser, APC, and cryotherapy (69,77).
APC
The use of APC began fairly recently within the realm of interventional pulmonology. The technique utilizes argon, a colorless, odorless, and chemically inert gas to pass down the probe (4). An ionized monopolar current is formed with argon gas and a high-voltage electrical field, targeting tissue and subsequently causing tissue coagulation or hemostasis (69) (Figure 4).
A variety of probe styles exist to optimize energy delivery depending on the anatomy of the MCAO and position of the bronchoscope that best achieves visualization (52). As with electrocautery, the power setting, during of activation, and probe distance will delineate the type of tissue effect APC will have.
As penetration is relatively minimal, this modality provides coagulation and good control of hemorrhage (4,70,78,79). APC has the ability for hemostasis even if the precise origin of bleed is not known (23,70,78,80).
Although highly effective in coagulation or granulation tissue debridement, the superficial penetration does not allow for effective vaporization or large scale tumor debulking (53). A risk unique to APC is argon gas embolism into the systemic circulation due to high gas pressure (69,81-83). Keeping flow to less than 0.80 L/minute has been shown to minimize the risk of gas embolism (84). Akin to other thermal modalities, other risks include airway fire and airway perforation.
Indirect therapeutic modalities
Probe cryotherapy
Cryotherapy, a cold ablative therapy, is able to destroy tumor tissues through the use of cryogenic liquid gas (N2O, N2, CO2) utilizing a pattern of freeze-thaw cycles (41,85-88). A cryogen is defined as a substance used to produce very low temperatures. As gas is released from high pressure to the tip of a flexible or rigid probe, it expands and thus creates rapid cooling of the distal tip by the Joule-Thompson effect (26). The degrees of temperature variation depend on the cryogen gas utilized. Low temperatures result in the immediate effect of dehydration and cellular crystallization as well as delayed effects of apoptosis and ischemia due to microthrombi formation (8,41,89-92).
The cryoprobe can be used either through flexible or rigid bronchoscopy (8). It is recommended to have at least 3 to 5 repeated 60-second freeze-thaw cycles to the lesion to facilitate increased cellular damage (8,93,94). After the last completed cycle, the probe is retracted from the tissue, and placed several millimeters adjacent to the site for another three cycles, creating an overlap of treated area (86,95). Necrotic tissue can be removed on a subsequent bronchoscopy (86,96).
Advantages of cryotherapy include the relatively low cost, virtually no risk of delayed stenosis, and positive hemostatic effect (88,92,97). Cartilage-like tissue, collagen, and poorly vascularized tissue are cryoresistant, and in turn decrease the risk of airway perforation, making cryotherapy one of the safest ablation techniques (8). Disadvantages include its delayed effects as stated previously, as well as limited depth of its cytotoxic action (8). The safety and efficacy of the therapy, incorporating factors such as successful removal of the obstruction, symptomatic improvement, and level of complications, indicate a highly effective therapeutic modality in MCAOs (26,96,98-104).
Cryospray is a related technology where liquid nitrogen (N2) is rapidly forced through a disposable catheter that fits down the working channel of a flexible bronchoscope. The cryogen will spray directly on the tissue to cause an extreme cooling effect with passive thawing. The rapid expansion of such compressed gas requires an open ventilator circuit to avoid pneumothorax or other barotrauma. Preliminary studies are promising but the technique has not been widely described in large cohorts (26).
Photodynamic therapy (PDT)
Since 1911, porphyrin-based photosensitizers have been extensively researched for its utility in providing non-thermal laser light to cause a phototoxic reaction which leads to cell death (23,89,105,106). This process is called PDT, with porfirmer sodium (Photofrin) currently being the most widely used agent (8,53,107,108). The mechanism involves direct cell damage by singlet oxygen, apoptosis, and indirect effect due to vascular stasis, inflammation, and immune response (8,89).
Intravenous photosensitizer is injected in the systemic circulation that is the absorbed mostly by metabolically active cells including malignant cells, skin, liver, and spleen (109). A light probe is inserted through a flexible bronchoscope, activated at the target lesion to a specific wavelength to achieve a penetration depth of 5–10 mm (24,110). A repeat bronchoscopy is required to clean debris and necrotic tissue 72 hours after light therapy, with repeat PDT cycles performed if needed (23,24,54,111). To limit toxicity, light therapy is performed approximately 48–72 hours after administration of photosensitizer to ensure clearance out of most normal tissue. Patients are advised to avoid light exposure due to partial retention of the compound for approximately 6 weeks (112).
As PDT is a non-thermal ablative therapy, its use is advantageous in patients requiring higher oxygen demands, as the risk of airway fire is negligible compared to thermal therapies. Disadvantages of the therapy consist of photosensitivity, mucosal sloughing causing subsequent respiratory failure, repeated bronchoscopies to evacuate debris, as well as hemoptysis, bronchitis, pneumonias, and severe endotracheal candidiasis (113,114). Our practice is to observe patients for 48 hours post treatment with a repeat bronchoscopy to clear debris before discharge. An additional disadvantage is its high cost (23,85,111). The delayed effect of the therapy essentially rules out the ability of immediate support in imminent respiratory failure.
Brachytherapy
Brachytherapy is an indirect therapeutic option using iridium-192 beads to provide localized radiation therapy within or alongside a tumor in the airway with the assistance of graduated radiopaque catheter and bronchoscope (8,23,53). The gamma radiation emitted through brachytherapy does not cause direct killing of cells, but rather causes breaks within DNA and therefore lead to apoptosis and decreased cell proliferation (89,91).
Unfortunately, at this time, patient selection remains ambiguous, as there is lack of evidence to support dose-rate methods or prediction of tumor response (4).
Clinical evidence of efficacy
Bronchoscopic interventions are often safer, promote superior cost effectiveness, and pose a better benefit to risk profile compared to open surgical options (20). These therapies are known to be effective in MCAO with further evidence showing it is the preferred method of palliative relief (27,59,68,115-117).
Studies have indicated significant improvement of 6-minute walk test, FEV1, and FVC, dyspnea, and Quality of Life (QoL) by day 30 of post-intervention (17,59,118). Technical success rate has been recorded to be approximately 88–100%, while procedural related complications were recorded to be 3% to 20.4% and mortality of 1–3.1% (17,68,115,116,119-123). Stenting was in fact, recorded to have better success rates compared to ablative techniques (115).
While complications and mortality rates vary, the patient population receiving interventions are frequently those with late-stage cancer and no further options for targeted treatment (123). The type of obstruction, extent, location, mechanism, symptoms, and stability of the patient need to be accounted for prior to choosing intervention (1,2,115,124). Utilizing rigid bronchoscopy for airway stabilization, followed by direct therapies such as mechanical debulking, thermal tools, cryorecanalization, and cryotherapy to combat endoluminal obstruction, with airway stenting to maintain airway patency when feasible, are an accepted approach for rapid restoration of the airway (8). Delayed bronchoscopic measures such as PDT and brachytherapy in addition to therapies used for rapid restoration, can also be utilized in non-emergent obstructions (4). Overall, evidence supports the use of multimodality and multidisciplinary approach that focuses on a combination of interventions rather than just one in order to produce successful results (125,126).
Future directions
As we move into the future, new modalities give hope to additional effective and precise options while aiming to decrease risks and complications. New directions include but not limited to intratumoral chemotherapy (ITC) and transbronchial needle injection (TBNI), drug eluting and biodegradable stents.
ITC and TBNI are two new modalities that are showing promise in reducing tumor size, and improving airway lumen (127). Through the use of endobronchial ultrasonography (EBUS), needle catheters are used for drug delivery directly into luminal tumors or lymph nodes (128). Currently, a new micro-infusion device, Blowfish™ (Mercator MedSystems, Emeryville, CA, USA) is being investigated for its utility (128). Drugs being examined include, but are not limited to, cisplatin, 5-fluorouracil, bleomycin, carboplatin, para-toluenesulfonamide, and paclitaxel (128-131). Advantages of these modalities include better precision of drug delivery and higher tumor concentration (132). There remains mixed evidence on systemic effects and toxicity such as neutropenia in patients exposed to the intratumoral injection at this time (133). No other major side effects have been noted (128). Evidence demonstrates ITC and TBNI as a feasible therapeutic option in a multimodal approach of restoring airway patency in MCAO, although further studies on drug of choice and modality are warranted (133-136).
Thus far, endeavors towards drug eluting airway stents remains suboptimal and may in part be due to other bronchoscopic modalities already available for palliation in patients with MCAO and concurrent low performance status (137). Therefore, as we approach the idea of airway drug eluting stents, prerequisites should be met in order to make the most ideal drug eluting airway stent. The stent should provide sufficient strength to maintain airway patency, be biocompatible so mucosal irritation will be negligible, have the ability to be biodegradable in order to prevent necessity of removal, and finally, provide drug therapy that in an effective manner (137).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Douglas Kyle Hogarth and Jonathan S. Kurman) for the series “Interventional Pulmonology and Advanced Bronchoscopy” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.11.09). The series “Interventional Pulmonology and Advanced Bronchoscopy” was commissioned by the editorial office without any funding or sponsorship. DMD reports personal fees from Boston Scientific, from null, outside the submitted work; The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Freitag L, Ernst A, Unger M, et al. A proposed classification system of central airway stenosis. Eur Respir J 2007;30:7-12. [Crossref] [PubMed]
- Murgu SD, Egressy K, Laxmanan B, et al. Central Airway Obstruction: Benign Strictures, Tracheobronchomalacia, and Malignancy-related Obstruction. Chest 2016;150:426-41. [Crossref] [PubMed]
- Semaan R, Yarmus L. Rigid bronchoscopy and silicone stents in the management of central airway obstruction. J Thorac Dis 2015;7:S352-62. [PubMed]
- Mudambi L, Miller R, Eapen GA. Malignant central airway obstruction. J Thorac Dis 2017;9:S1087-S1110. [Crossref] [PubMed]
- Stöhr S, Bolliger CT. Stents in the management of malignant airway obstruction. Monaldi Arch Chest Dis 1999;54:264-8. [PubMed]
- Ginsberg RJ, Vokes EE, Raben A. Cancer of the lung, section 2: non-small cell lung cancer. Cancer: Principles & Practice, 1997.
- Theodore PR. Emergent management of malignancy-related acute airway obstruction. Emerg Med Clin North Am 2009;27:231-41. [Crossref] [PubMed]
- Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med 2004;169:1278-97 [Crossref] [PubMed]
- Gaissert HA, Grillo HC, Shadmehr MB, et al. Uncommon primary tracheal tumors. Ann Thorac Surg 2006;82:268-72; discussion 272. [Crossref] [PubMed]
- Hemminki K, Li X. Incidence trends and risk factors of carcinoid tumors: a nationwide epidemiologic study from Sweden. Cancer 2001;92:2204-10. [Crossref] [PubMed]
- Marchioni A, Lasagni A, Busca A, et al. Endobronchial metastasis: an epidemiologic and clinicopathologic study of 174 consecutive cases. Lung Cancer 2014;84:222-8. [Crossref] [PubMed]
- Jabbardarjani H, Herth F, Kiani A, et al. Central Airway Obstruction Masquerading as Difficult-to-Treat Asthma: A Retrospective Study. J Bronchology Interv Pulmonol 2009;16:6-9. [Crossref] [PubMed]
- Braman SS, Whitcomb ME. Endobronchial metastasis. Arch Intern Med 1975;135:543-7. [Crossref] [PubMed]
- Abers MS, Sandvall BP, Sampath R, et al. Postobstructive pneumonia: an underdescribed syndrome. Clin Infect Dis 2016;62:957-61. [Crossref] [PubMed]
- Henschke CI, Lee I-J, Wu N, et al. CT screening for lung cancer: prevalence and incidence of mediastinal masses. Radiology 2006;239:586-90. [Crossref] [PubMed]
- Ducrocq X, Thomas P, Massard G, et al. Operative risk and prognostic factors of typical bronchial carcinoid tumors. Ann Thorac Surg 1998;65:1410-4. [Crossref] [PubMed]
- Stratakos G, Gerovasili V, Dimitropoulos C, et al. Survival and Quality of Life Benefit after Endoscopic Management of Malignant Central Airway Obstruction. J Cancer 2016;7:794-802. [Crossref] [PubMed]
- Folch E, Keyes C. Airway stents. Ann Cardiothorac Surg 2018;7:273-83. [Crossref] [PubMed]
- Seijo LM, Sterman DH. Interventional pulmonology. N Engl J Med 2001;344:740-9. [Crossref] [PubMed]
- Vonk-Noordegraaf A, Postmus PE, Sutedja TG. Bronchoscopic treatment of patients with intraluminal microinvasive radiographically occult lung cancer not eligible for surgical resection: a follow-up study. Lung Cancer 2003;39:49-53. [Crossref] [PubMed]
- Wood DE. Management of malignant tracheobronchial obstruction. Surg Clin North Am 2002;82:621-42. [Crossref] [PubMed]
- Daddi G, Puma F, Avenia N, et al. Resection with curative intent after endoscopic treatment of airway obstruction. Ann Thorac Surg 1998;65:203-7. [Crossref] [PubMed]
- Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J 2006;27:1258-71. [Crossref] [PubMed]
- Bilaçeroğlu S. Endobronchial Ablative Therapies. Clin Chest Med 2018;39:139-48. [Crossref] [PubMed]
- Ayers ML, Beamis JF. Rigid bronchoscopy in the twenty-first century. Clin Chest Med 2001;22:355-64. [Crossref] [PubMed]
- DiBardino DM, Lanfranco AR, Haas AR. Bronchoscopic cryotherapy. clinical applications of the cryoprobe, cryospray, and cryoadhesion. Annals of the American Thoracic Society 2016;13:1405-15. [Crossref] [PubMed]
- Guibert N, Mhanna L, Droneau S, et al. Techniques of endoscopic airway tumor treatment. J Thorac Dis 2016;8:3343-60. [Crossref] [PubMed]
- Sunna R. Cryotherapy and Cryodebridement. In: Ernst A, Herth FJ. editors. Principles and Practice of Interventional Pulmonology. New York, NY: Springer New York, 2013:343-50.
- Hetzel M, Hetzel J, Schumann C, et al. Cryorecanalization: a new approach for the immediate management of acute airway obstruction. J Thorac Cardiovasc Surg 2004;127:1427-31. [Crossref] [PubMed]
- Franke KJ, Szyrach M, Nilius G, et al. Experimental study on biopsy sampling using new flexible cryoprobes: influence of activation time, probe size, tissue consistency, and contact pressure of the probe on the size of the biopsy specimen. Lung 2009;187:253-9. [Crossref] [PubMed]
- Ma Q, Shi B, Tian Y, et al. Fibrobronchoscopic cryosurgery for secondary malignant tumors of the trachea and main bronchi. Thorac Cancer 2016;7:459-66. [Crossref] [PubMed]
- Casal RF, Iribarren J, Eapen G, et al. Safety and effectiveness of microdebrider bronchoscopy for the management of central airway obstruction. Respirology 2013;18:1011-5. [Crossref] [PubMed]
- Lunn W, Garland R, Ashiku S, et al. Microdebrider bronchoscopy: a new tool for the interventional bronchoscopist. Ann Thorac Surg 2005;80:1485-8. [Crossref] [PubMed]
- Wood DE, Liu YH, Vallières E, et al. Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg 2003;76:167-72; discussion 173. [Crossref] [PubMed]
- Godoy MCB, Saldana DA, Rao PP, et al. Multidetector CT evaluation of airway stents: what the radiologist should know. Radiographics 2014;34:1793-806. [Crossref] [PubMed]
- Mehta AC, Dasgupta A. Airway stents. Clin Chest Med 1999;20:139-51. [Crossref] [PubMed]
- Saad CP, Murthy S, Krizmanich G, et al. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest 2003;124:1993-9. [Crossref] [PubMed]
- Colt HG, Dumon JF. Airway stents. Present and future. Clin Chest Med 1995;16:465-78. [PubMed]
- Miyazawa T, Yamakido M, Ikeda S, et al. Implantation of ultraflex nitinol stents in malignant tracheobronchial stenoses. Chest 2000;118:959-65. [Crossref] [PubMed]
- Hauck RW, Lembeck RM, Emslander HP, et al. Implantation of Accuflex and Strecker stents in malignant bronchial stenoses by flexible bronchoscopy. Chest 1997;112:134-44. [Crossref] [PubMed]
- Dasgupta A, Dolmatch BL, Abi-Saleh WJ, et al. Self-expandable metallic airway stent insertion employing flexible bronchoscopy: preliminary results. Chest 1998;114:106-9. [Crossref] [PubMed]
- Susanto I, Peters JI, Levine SM, et al. Use of balloon-expandable metallic stents in the management of bronchial stenosis and bronchomalacia after lung transplantation. Chest 1998;114:1330-5. [Crossref] [PubMed]
- Freitag L, Tekolf E, Steveling H, et al. Management of malignant esophagotracheal fistulas with airway stenting and double stenting. Chest 1996;110:1155-60. [Crossref] [PubMed]
- Noppen M, Poppe K, D’Haese J, et al. Interventional bronchoscopy for treatment of tracheal obstruction secondary to benign or malignant thyroid disease. Chest 2004;125:723-30. [Crossref] [PubMed]
- Monnier P, Mudry A, Stanzel F, et al. The use of the covered Wallstent for the palliative treatment of inoperable tracheobronchial cancers. A prospective, multicenter study. Chest 1996;110:1161-8. [Crossref] [PubMed]
- Husain SA, Finch D, Ahmed M, et al. Long-term follow-up of ultraflex metallic stents in benign and malignant central airway obstruction. Ann Thorac Surg 2007;83:1251-6. [Crossref] [PubMed]
- Boiselle PM, Ernst A. Recent advances in central airway imaging. Chest 2002;121:1651-60. [Crossref] [PubMed]
- Puma F, Farabi R, Urbani M, et al. Long-term safety and tolerance of silicone and self-expandable airway stents: an experimental study. Ann Thorac Surg 2000;69:1030-4. [Crossref] [PubMed]
- Lemaire A, Burfeind WR, Toloza E, et al. Outcomes of tracheobronchial stents in patients with malignant airway disease. Ann Thorac Surg 2005;80:434-7; discussion 437. [Crossref] [PubMed]
- Lin SM, Lin TY, Chou CL, et al. Metallic stent and flexible bronchoscopy without fluoroscopy for acute respiratory failure. Eur Respir J 2008;31:1019-23. [Crossref] [PubMed]
- Dumon JF. A dedicated tracheobronchial stent. Chest 1990;97:328-32. [Crossref] [PubMed]
- Mahmood K, Wahidi MM. Ablative therapies for central airway obstruction. Semin Respir Crit Care Med 2014;35:681-92. [Crossref] [PubMed]
- Du Rand IA, Barber PV, Goldring J, et al. British Thoracic Society guideline for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax 2011;66:iii1-21. [PubMed]
- Seaman JC, Musani AI. Endobronchial ablative therapies. Clin Chest Med 2013;34:417-25. [Crossref] [PubMed]
- Dunlap DG, Ravenel J, Sechrist J, et al. Interventional therapies for central airways. J Thorac Imaging 2019;34:W49-W59. [Crossref] [PubMed]
- Dumon JF, Reboud E, Garbe L, et al. Treatment of tracheobronchial lesions by laser photoresection. Chest 1982;81:278-84. [Crossref] [PubMed]
- Cavaliere S, Foccoli P, Farina PL. Nd:YAG laser bronchoscopy. A five-year experience with 1,396 applications in 1,000 patients. Chest 1988;94:15-21. [Crossref] [PubMed]
- Mehta AC, Lee FY, Cordasco EM, et al. Concentric tracheal and subglottic stenosis. Management using the Nd-YAG laser for mucosal sparing followed by gentle dilatation. Chest 1993;104:673-7. [Crossref] [PubMed]
- Amjadi K, Voduc N, Cruysberghs Y, et al. Impact of interventional bronchoscopy on quality of life in malignant airway obstruction. Respiration 2008;76:421-8. [Crossref] [PubMed]
- Khan S, Mehta A. Endobronchial Laser Therapy. Semin Respir Crit Care Med 1997;18:525-34. [Crossref]
- Ramser ER, Beamis JF. Laser bronchoscopy. Clin Chest Med 1995;16:415-26. [PubMed]
- Cavaliere S, Venuta F, Foccoli P, et al. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest 1996;110:1536-42. [Crossref] [PubMed]
- Dumon JF, Shapshay S, Bourcereau J, et al. Principles for safety in application of neodymium-YAG laser in bronchology. Chest 1984;86:163-8. [Crossref] [PubMed]
- Brutinel WM, Cortese DA, McDougall JC, et al. A two-year experience with the neodymium-YAG laser in endobronchial obstruction. Chest 1987;91:159-65. [Crossref] [PubMed]
- Kvale PA, Eichenhorn MS, Radke JR, et al. YAG laser photoresection of lesions obstructing the central airways. Chest 1985;87:283-8. [Crossref] [PubMed]
- Dalupang JJ, Shanks TG, Colt HG. Nd-YAG laser damage to metal and silicone endobronchial stents: delineation of margins of safety using an in vitro experimental model. Chest 2001;120:934-40. [Crossref] [PubMed]
- Mughal MM, Gildea TR, Murthy S, et al. Short-term deployment of self-expanding metallic stents facilitates healing of bronchial dehiscence. Am J Respir Crit Care Med 2005;172:768-71. [Crossref] [PubMed]
- Chen C-H, Wu B-R, Cheng W-C, et al. Interventional pulmonology for patients with central airway obstruction: An 8-year institutional experience. Medicine 2017;96:e5612 [Crossref] [PubMed]
- Sheski FD, Mathur PN. Endobronchial electrosurgery: argon plasma coagulation and electrocautery. Semin Respir Crit Care Med 2004;25:367-74. [Crossref] [PubMed]
- Tremblay A, Marquette CH. Endobronchial electrocautery and argon plasma coagulation: a practical approach. Can Respir J 2004;11:305-10. [Crossref] [PubMed]
- Tremblay A, Michaud G, Urbanski SJ. Hot biopsy forceps in the diagnosis of endobronchial lesions. Eur Respir J 2007;29:108-11. [Crossref] [PubMed]
- Horinouchi H, Miyazawa T, Takada K, et al. Safety study of endobronchial electrosurgery for tracheobronchial lesions. Journal of Bronchology 2008;15:228-32. [Crossref]
- Mahajan AK, Herdina KA, Howk KA. Performance of a Novel Electrosurgical Device for Cutting and Coagulation of Central Airway Obstructions. THE THREE" I’s" OF 2018.
- Boxem Tv, Muller M, Venmans B, et al. Nd-YAG laser vs bronchoscopic electrocautery for palliation of symptomatic airway obstruction: a cost-effectiveness study. Chest 1999;116:1108-12. [Crossref] [PubMed]
- van Boxem TJ, Westerga J, Venmans BJ, et al. Tissue effects of bronchoscopic electrocautery: bronchoscopic appearance and histologic changes of bronchial wall after electrocautery. Chest 2000;117:887-91. [Crossref] [PubMed]
- Wahidi MM, Unroe MA, Adlakha N, et al. The use of electrocautery as the primary ablation modality for malignant and benign airway obstruction. J Thorac Oncol 2011;6:1516-20. [Crossref] [PubMed]
- Sachdeva A, Pickering EM, Lee HJ. From electrocautery, balloon dilatation, neodymium-doped:yttrium-aluminum-garnet (Nd:YAG) laser to argon plasma coagulation and cryotherapy. J Thorac Dis 2015;7:S363-79. [PubMed]
- Morice RC, Ece T, Ece F, et al. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001;119:781-7. [Crossref] [PubMed]
- Okada S, Yamauchi H, Ishimori S, et al. Endoscopic surgery with a flexible bronchoscope and argon plasma coagulation for tracheobronchial tumors. J Thorac Cardiovasc Surg 2001;121:180-2. [Crossref] [PubMed]
- Nihei K, Ishikura S, Kawashima M, et al. Short-course palliative radiotherapy for airway stenosis in non-small cell lung cancer. Int J Clin Oncol 2002;7:284-8. [Crossref] [PubMed]
- Reichle G, Freitag L, Kullmann HJ, et al. Argon plasma coagulation in bronchology: a new method--alternative or complementary? Pneumologie 2000;54:508-16. [Crossref] [PubMed]
- Reddy C, Majid A, Michaud G, et al. Gas embolism following bronchoscopic argon plasma coagulation: a case series. Chest 2008;134:1066-9. [Crossref] [PubMed]
- Shaw Y, Yoneda KY, Chan AL. Cerebral gas embolism from bronchoscopic argon plasma coagulation: a case report. Respiration 2012;83:267-70. [Crossref] [PubMed]
- Feller-Kopman D, Lukanich JM, Shapira G, et al. Gas flow during bronchoscopic ablation therapy causes gas emboli to the heart: a comparative animal study. Chest 2008;133:892-6. [Crossref] [PubMed]
- Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society/American Thoracic Society. Eur Respir J 2002;19:356-73. [PubMed]
- Mathur PN, Wolf KM, Busk MF, et al. Fiberoptic bronchoscopic cryotherapy in the management of tracheobronchial obstruction. Chest 1996;110:718-23. [Crossref] [PubMed]
- Usuda J, Ichinose S, Ishizumi T, et al. Outcome of photodynamic therapy using NPe6 for bronchogenic carcinomas in central airways >1.0 cm in diameter. Clin Cancer Res 2010;16:2198-204. [Crossref] [PubMed]
- Corti L, Toniolo L, Boso C, et al. Long-term survival of patients treated with photodynamic therapy for carcinoma in situ and early non-small-cell lung carcinoma. Lasers Surg Med 2007;39:394-402. [Crossref] [PubMed]
- Vergnon JM, Huber RM, Moghissi K. Place of cryotherapy, brachytherapy and photodynamic therapy in therapeutic bronchoscopy of lung cancers. Eur Respir J 2006;28:200-18. [Crossref] [PubMed]
- Asimakopoulos G, Beeson J, Evans J, et al. Cryosurgery for malignant endobronchial tumors: analysis of outcome. Chest 2005;127:2007-14. [Crossref] [PubMed]
- Vergnon JM, Schmitt T, Alamartine E, et al. Initial combined cryotherapy and irradiation for unresectable non-small cell lung cancer. Preliminary results. Chest 1992;102:1436-40. [Crossref] [PubMed]
- Deygas N, Froudarakis M, Ozenne G, et al. Cryotherapy in early superficial bronchogenic carcinoma. Chest 2001;120:26-31. [Crossref] [PubMed]
- Gage AA, Guest K, Montes M, et al. Effect of varying freezing and thawing rates in experimental cryosurgery. Cryobiology 1985;22:175-82. [Crossref] [PubMed]
- Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology 1977;14:251-72. [Crossref] [PubMed]
- Maiwand M, Mathur P. Endobronchial Cryotherapy. Semin Respir Crit Care Med 1997;18:545-54. [Crossref]
- Homasson JP, Renault P, Angebault M, et al. Bronchoscopic cryotherapy for airway strictures caused by tumors. Chest 1986;90:159-64. [Crossref] [PubMed]
- McCaughan JS, Hawley PC, Brown DG, et al. Effect of light dose on the photodynamic destruction of endobronchial tumors. Ann Thorac Surg 1992;54:705-11. [Crossref] [PubMed]
- Walsh DA, Maiwand MO, Nath AR, et al. Bronchoscopic cryotherapy for advanced bronchial carcinoma. Thorax 1990;45:509-13. [Crossref] [PubMed]
- Maiwand MO, Homasson JP. Cryotherapy for tracheobronchial disorders. Clin Chest Med 1995;16:427-43. [PubMed]
- Rodgers BM, Moazam F, Talbert JL. Endotracheal cryotherapy in the treatment of refractory airway strictures. Ann Thorac Surg 1983;35:52-7. [Crossref] [PubMed]
- Marasso A, Gallo E, Massaglia GM, et al. Cryosurgery in bronchoscopic treatment of tracheobronchial stenosis. Indications, limits, personal experience. Chest 1993;103:472-4. [Crossref] [PubMed]
- Maiwand MO. Cryotherapy for advanced carcinoma of the trachea and bronchi. Br Med J (Clin Res Ed) 1986;293:181-2. [Crossref] [PubMed]
- Maiwand MO. The role of cryosurgery in palliation of tracheo-bronchial carcinoma. Eur J Cardiothorac Surg 1999;15:764-8. [Crossref] [PubMed]
- Maiwand MO, Evans JM, Beeson JE. The application of cryosurgery in the treatment of lung cancer. Cryobiology 2004;48:55-61. [Crossref] [PubMed]
- Hennequin C, Tredaniel J, Chevret S, et al. Predictive factors for late toxicity after endobronchial brachytherapy: a multivariate analysis. Int J Radiat Oncol Biol Phys 1998;42:21-7. [Crossref] [PubMed]
- Speiser BL, Spratling L. Remote afterloading brachytherapy for the local control of endobronchial carcinoma. Int J Radiat Oncol Biol Phys 1993;25:579-87. [Crossref] [PubMed]
- Moghissi K, Dixon K, Thorpe JAC, et al. Photodynamic therapy (PDT) in early central lung cancer: a treatment option for patients ineligible for surgical resection. Thorax 2007;62:391-5. [Crossref] [PubMed]
- Allison R, Moghissi K, Downie G, et al. Photodynamic therapy (PDT) for lung cancer. Photodiagnosis Photodyn Ther 2011;8:231-9. [Crossref] [PubMed]
- Gomer CJ, Dougherty TJ. Determination of [3H]- and [14C]hematoporphyrin derivative distribution in malignant and normal tissue. Cancer Res 1979;39:146-51. [PubMed]
- Dougherty TJ, Marcus SL. Photodynamic therapy. Eur J Cancer 1992;28A:1734-42. [Crossref] [PubMed]
- Du Rand IA, Barber PV, Goldring J, et al. British Thoracic Society Interventional Bronchoscopy Guideline G. British Thoracic Society guideline for advanced diagnostic and therapeutic flexible. Thorax 2011;66:iii1-21. [PubMed]
- Edell ES, Cortese DA. Photodynamic therapy. Its use in the management of bronchogenic carcinoma. Clin Chest Med 1995;16:455-63. [PubMed]
- Maziak DE, Markman BR, MacKay JA, et al. Photodynamic therapy in nonsmall cell lung cancer: a systematic review. Ann Thorac Surg 2004;77:1484-91. [Crossref] [PubMed]
- Triesscheijn M, Baas P, Schellens JHM, et al. Photodynamic therapy in oncology. Oncologist 2006;11:1034-44. [Crossref] [PubMed]
- Ost DE, Ernst A, Grosu HB, et al. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest 2015;147:1282-98. [Crossref] [PubMed]
- Jeon K, Kim H, Yu CM, et al. Rigid bronchoscopic intervention in patients with respiratory failure caused by malignant central airway obstruction. J Thorac Oncol 2006;1:319-23. [Crossref] [PubMed]
- Mitchell PD, Kennedy MP. Bronchoscopic management of malignant airway obstruction. Adv Ther 2014;31:512-38. [Crossref] [PubMed]
- Oviatt PL, Stather DR, Michaud G, et al. Exercise capacity, lung function, and quality of life after interventional bronchoscopy. J Thorac Oncol 2011;6:38-42. [Crossref] [PubMed]
- Ost DE, Ernst A, Grosu HB, et al. Complications following therapeutic bronchoscopy for malignant central airway obstruction: results of the aquire registry. Chest 2015;148:450-71. [Crossref] [PubMed]
- Razi SS, Lebovics RS, Schwartz G, et al. Timely airway stenting improves survival in patients with malignant central airway obstruction. Ann Thorac Surg 2010;90:1088-93. [Crossref] [PubMed]
- Guibert N, Mazieres J, Lepage B, et al. Prognostic factors associated with interventional bronchoscopy in lung cancer. Ann Thorac Surg 2014;97:253-9. [Crossref] [PubMed]
- Dalar L, Özdemir C, Abul Y, et al. Therapeutic bronchoscopic interventions for malignant airway obstruction: A retrospective study from experience on 547 patients. Medicine 2016;95:e3886 [Crossref] [PubMed]
- Shin B, Chang B, Kim H, et al. Interventional bronchoscopy in malignant central airway obstruction by extra-pulmonary malignancy. BMC Pulm Med 2018;18:46. [Crossref] [PubMed]
- Gasparini S, Bonifazi M. Management of endobronchial tumors. Curr Opin Pulm Med 2016;22:245-51. [Crossref] [PubMed]
- Brichet A, Verkindre C, Dupont J, et al. Multidisciplinary approach to management of postintubation tracheal stenoses. Eur Respir J 1999;13:888-93. [Crossref] [PubMed]
- Jones LM, Mair EA, Fitzpatrick TM, et al. Multidisciplinary airway stent team: a comprehensive approach and protocol for tracheobronchial stent treatment. Ann Otol Rhinol Laryngol 2000;109:889-98. [Crossref] [PubMed]
- Yarmus L, Mallow C, Akulian J, et al. Prospective multicentered safety and feasibility pilot for endobronchial intratumoral chemotherapy. Chest 2019;156:562-70. [Crossref] [PubMed]
- Mohan A, Harris K, Bowling MR, et al. Therapeutic bronchoscopy in the era of genotype directed lung cancer management. J Thorac Dis 2018;10:6298-309. [Crossref] [PubMed]
- Celikoğlu SI, Karayel T, Demirci S, et al. Direct injection of anti-cancer drugs into endobronchial tumours for palliation of major airway obstruction. Postgrad Med J 1997;73:159-62. [Crossref] [PubMed]
- Liu M, Ma P, Lu Z. Local chemotherapy by fibrobronchoscopy for advanced bronchogenic carcinoma. Zhonghua Jie He He Hu Xi Za Zhi 2000;23:550-1. [PubMed]
- Li SY, Li Q, Guan WJ, et al. Effects of para-toluenesulfonamide intratumoral injection on non-small cell lung carcinoma with severe central airway obstruction: A multi-center, non-randomized, single-arm, open-label trial. Lung Cancer 2016;98:43-50. [Crossref] [PubMed]
- Celikoglu F, Celikoglu SI, Goldberg EP. Bronchoscopic intratumoral chemotherapy of lung cancer. Lung Cancer 2008;61:1-12. [Crossref] [PubMed]
- Hohenforst-Schmidt W, Zarogoulidis P, Darwiche K, et al. Intratumoral chemotherapy for lung cancer: re-challenge current targeted therapies. Drug Des Devel Ther 2013;7:571-83. [PubMed]
- Mehta HJ, Begnaud A, Penley AM, et al. Restoration of patency to central airways occluded by malignant endobronchial tumors using intratumoral injection of cisplatin. Annals of the American Thoracic Society 2015;12:1345-50. [Crossref] [PubMed]
- Celikoglu SI, Celikoglu F, Goldberg EP. Endobronchial intratumoral chemotherapy (EITC) followed by surgery in early non-small cell lung cancer with polypoid growth causing erroneous impression of advanced disease. Lung Cancer 2006;54:339-46. [Crossref] [PubMed]
- Celikoglu F, Celikoglu SI, York AM, et al. Intratumoral administration of cisplatin through a bronchoscope followed by irradiation for treatment of inoperable non-small cell obstructive lung cancer. Lung Cancer 2006;51:225-36. [Crossref] [PubMed]
- Hohenforst-Schmidt W, Zarogoulidis P, Pitsiou G, et al. Drug eluting stents for malignant airway obstruction: A critical review of the literature. J Cancer 2016;7:377-90. [Crossref] [PubMed]
Cite this article as: Pasricha VG, DiBardino DM, Ma KC. Management of malignant central airway obstruction. Shanghai Chest 2020;4:26.