
Multiport video-assisted thoracoscopic surgery pneumonectomy
Introduction
Pneumonectomy has evolved throughout the history of surgery from a primary treatment of lung cancer and infectious diseases to a procedure of last resort. The first successful one stage pneumonectomy for lung cancer is commonly attributed to Evarts Graham in 1933. In his original publication, Graham himself acknowledged Hermann Kümmell as performing the first pneumonectomy for cancer in 1911, but that patient did not survive (1). The first video-assisted thoracoscopic lobectomy for lung cancer was described by Giancarlo Rovario in 1991 (2). Compared to laparoscopic surgery, video-assisted thoracoscopic surgery (VATS) surgery has gained slower but nevertheless steady adoption over the last few decades. The Society of Thoracic Surgery database now shows that 55% (13,147/23,882) of all lobectomies performed in the database between 2010 and 2013 were done via VATS, compared to approximately 15% (2,557/16,732) in the European Society of Thoracic Surgery database during the same time period (3). Adoption for VATS pneumonectomy has been considerably slower, with data during the same time period showing that only 5% (72/1,338) of pneumonectomies in the STS database were performed via VATS and only 1% (27/2,276) in the ESTS database (3,4). The reasons for this slower adoption are likely multiple, including surgeons’ understandable fear of injury at the level of the main pulmonary artery, difficult hilar dissection in central tumors, and problems with both retracting and extracting larger specimens. Nevertheless, data shows it is possible to achieve an oncologically equivalent or even superior operation (average of 22 lymph nodes dissected with VATS compared to 13 with open) with the potential for similar 30- and 90-day mortality and overall 5-year survival (5-8). Furthermore, there are data that VATS may lead to reduced acute pain and increased chance of being pain free at 1 year after pneumonectomy compared to open thoracotomy (5).
Given the comparatively high mortality rate associated with pneumonectomy (5% at 30 days, compared to 1.3% for lobectomy) and in the interest of maximizing the patient’s pulmonary reserve and overall functional status, it is our practice to routinely perform a parenchymal-sparing operation whenever possible, such as sleeve lobectomy with or without pulmonary arthroplasty. When that is not possible, VATS pneumonectomy is indicated, with conversion to open thoracotomy if needed.
Preoperative testing
Standard preoperative testing is identical to that for open pneumonectomy. Pulmonary functioning tests and echocardiography are performed routinely for all patients, and split lung perfusion scans may be performed as indicated. When cardiopulmonary reserve is borderline, cardiopulmonary exercise testing may be indicated. Assessment of right heart function including catheterization and testing for pulmonary hypertension are essential for optimal perioperative management. For patients who are older than 75 years of age, we routinely perform frailty testing as another objective measure of suitability for an operation.
Technique
Positioning for a VATS pneumonectomy is similar to that for VATS lobectomy, with patients placed on their side in flexion, with the ipsilateral arms rotated cephalad and held in place using arm boards. Port placement is also similar to port positions used for VATS lobectomy. We routinely place a 1 cm port at the 9th intercostal space at the posterior axillary line, a 1 cm port at the 7th intercostal space at the anterior axillary line, and a 4 cm access incision at the 4th intercostal space at the anterior axillary line. This access incision will be used for specimen retrieval at the end of the case. We avoid rib spreading whenever possible in order to maintain the postoperative pain advantage of the VATS technique over conventional thoracotomy. Alexis wound protectors (Allied Medical, Rancho Santa Margarita, CA, USA) of appropriate sizes may be helpful for allowing the placement of multiple instruments through each of these ports as needed without traumatizing the intercostal nerve bundle.
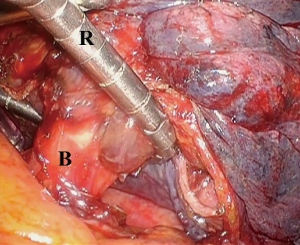
Exposure of the pulmonary hilum may be difficult in cases of bulky tumors which can make retraction with low profile thoracoscopic instruments very difficult. Retraction of the lung may be aided by used of the Diamond-Flex retractor (CareFusion, San Diego, CA, USA), placed around the pulmonary hilum. This retraction technique may be useful for pushing the bulky lung away from the mediastinum without risking parenchymal tearing with the associated bleeding. This aids with exposure of the critical hilar structures (Figure 1).
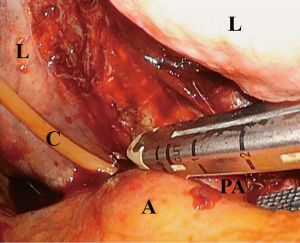
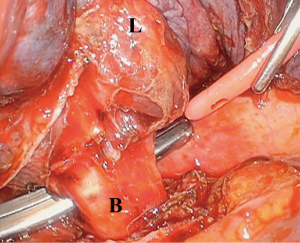
The operative steps for VATS pneumonectomy are similar to that of an open pneumonectomy. As with most VATS lobectomies, the lung is retracted cephalad and the inferior pulmonary ligament is divided with electrocautery and/or surgical sealing device. The inferior pulmonary vein is exposed and encircled with blunt dissection but not divided yet. The mediastinal pleura around the pulmonary hilum is then divided. The superior pulmonary vein is similarly encircled. Although division of the pulmonary artery before division of the pulmonary veins avoids potential engorgement of the specimen and the associated operative difficulties thereof, it is often not possible without division of the veins beforehand. For this reason, division of the veins should be delayed as long as possible and then performed in quick succession in order to move on to dissection and division of the main pulmonary artery. It is advisable to place the stapler across the artery and gently close it, observing for any hemodynamic changes associated with interruption of pulmonary blood flow (Figure 2). This is true especially for left sided pneumonectomy, by which too proximal division of the main pulmonary artery may obstruct blood flow through the main pulmonary artery and thus the contralateral pulmonary artery. The bronchus is typically divided last with a thick tissue load of an endoscopic stapler, attempting to divide it as reasonably close to the carina as possible to minimize the length of the bronchial stump and thus the risk of bronchopleural fistula (Figure 3). To minimize the risk of the aforementioned complication, we routinely reinforce the bronchial stump with autologous vascularized tissue, such as a thymic fat pad, intercostal muscle flap, or when an intrapericardial dissection is performed, via the divided posterior edge of pericardium.
Postoperative management
We routinely leave a chest tube in place at the end of the procedure, connected to a balanced drainage system in order to minimize the risk of early and excessive mediastinal shift. The drain is typically removed within the first 24–48 hours. Intravenous fluids minimized and discontinued as early as possible. Early ambulation and pulmonary toilet are of critical importance.
Pitfalls, tips, and tricks
As mentioned previously, we find it critical to routinely obtain control of both pulmonary veins individually, and then divide them in quick succession with a surgical stapler in order to minimize the potential for venous engorgement that can occur prior to exposure and division of the main pulmonary artery.
In cases of centrally located tumors, with significant adhesions, and/or after neoadjuvant chemotherapy or immunotherapy, dissecting the main pulmonary artery can be difficult. Dissection of the pulmonary artery away from the bronchus should be performed in a blunt fashion and with dissection towards the firm surface of the cartilaginous bronchus in order to minimize the risk of injury to the artery. With the artery encircled, we routinely used a red rubber catheter behind the vessel to guide the anvil portion of the endoscopic stapler through the window to minimize risk of injury to the artery wall during this critical step of the operation.
If difficulty is expected prior to this dissection, an alternate technique is to perform mediastinoscopy immediately before the pneumonectomy. In addition to the mediastinal staging information, it prevents unindicated resection in the setting of unsuspected mediastinal nodal disease. Mediastinoscopy also allows limited dissection of the plane between the main pulmonary artery and the mainstem bronchus making it easier to establish this dissection plane later at the time of VATS. Although this additional step adds time to the procedure, it may prove beneficial in minimizing the difficulty in dissection of these structures.
An alternative if not preferred approach to a difficult pulmonary hilar dissection is to open the pericardium and perform the dissection within the sac. The pericardium is opened in a longitudinal fashion anterior to the pulmonary hilum and the artery is encircled and divided at this level. This may be particularly useful for left sided dissections in which the proximal left main pulmonary artery is shorter, and the aortic arch may prevent easy and safe passage of the stapler. This intrapericardial dissection can also provide proximal vascular control such as via an umbilical tape snare to help allow for control in the event of pulmonary artery injury during the dissection.
In the event of large, bulky tumors, specimen extraction can also be challenging, and we attempt to avoid rib spreading whenever possible as it may negate some of the advantages of VATS. Alternative techniques to assist with specimen extraction include placing the access incision as anterior as possible to take advantage of where the rib spaces are the widest, further lengthening the access incision itself, or occasionally, resecting a segment of rib at the inferior aspect of the incision. Depending on surgeon preference, an extra-thoracic extraction, such as in the subxiphoid or subcostal region with or without partial detachment/division of the hemidiaphragm may allow for removal of the specimen through an area other than an intercostal space. The diaphragm is then repaired by fixating it to the overlying external oblique fascia using nonabsorbable heavy suture material.
Conclusions
Multiport VATS pneumonectomy, when performed in experienced centers on appropriate patients, is a safe and feasible alternative to conventional pneumonectomy via thoracotomy. With additional surgical experience and increasingly widespread comfort and utilization of VATS for all lung types of resections, VATS pneumonectomy will continue to gain more acceptance when indicated.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lorenzo Spaggiari and Luca Bertolaccini) for the series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: The series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” was commissioned by the editorial office without any funding or sponsorship. TLD, Medtronic consultant. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- D'Amico TA. Historical perspectives of The American Association for Thoracic Surgery: Evarts A. Graham (1883-1957). J Thorac Cardiovasc Surg 2011;142:735-9. [Crossref] [PubMed]
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Seder CW, Wright CD, Chang AC, et al. The Society of Thoracic Surgeons General Thoracic Surgery Database Update on Outcomes and Quality. Ann Thorac Surg 2016;101:1646-54. [Crossref] [PubMed]
- Begum S, Hansen HJ, Papagiannopoulos K. VATS anatomic lung resections-the European experience. J Thorac Dis 2014;6:S203-10. [PubMed]
- Battoo A, Jahan A, Yang Z, et al. Thoracoscopic pneumonectomy: an 11-year experience. Chest 2014;146:1300-9. [Crossref] [PubMed]
- Liu Y, Gao Y, Zhang H, et al. Video-assisted versus conventional thoracotomy pneumonectomy: a comparison of perioperative outcomes and short-term measures of convalescence. J Thorac Dis 2016;8:3537-42. [Crossref] [PubMed]
- Yang C, Yendamuri S, Mayne NR, et al. The role of thoracoscopic pneumonectomy in the management of non-small cell lung cancer: A multicenter study. J Thorac Cardiovasc Surg 2019;158:252-64.e2. [Crossref] [PubMed]
- Hennon MW, Kumar A, Devisetty H, et al. Minimally Invasive Approaches Do Not Compromise Outcomes for Pneumonectomy: A Comparison Using the National Cancer Database. J Thorac Oncol 2019;14:107-14. [Crossref] [PubMed]
Cite this article as: Jordan S, Hennon M, Demmy TL. Multiport video-assisted thoracoscopic surgery pneumonectomy. Shanghai Chest 2020;4:32.


