Management of airway complications following lung transplantation
Introduction
Airway complications (ACs) following lung transplantation have been a source of significant morbidity and mortality since the field began. The early incidence of ACs was 60% to 80%. With improvements in surgical techniques, organ preservation, immunosuppression, and medical management of the recipient, the incidence has decreased (1-6). Despite these advances, ACs continue to contribute to morbidity and mortality after lung transplantation. Even with treatment, ACs are at high likelihood of recurrence. Approximately one third of patients treated for ACs will develop a second AC (7).
ACs after lung transplantation include bronchial stenosis, necrosis, dehiscence, fistula formation, infections, excessive granulation tissue formation, and malacia.
This article provides a brief review of the incidence and classification of ACs and the risk factors for ACs. It then describes the spectrum of ACs which can occur after lung transplantation as well as current treatment strategies.
Incidence
There is a wide range of reported AC incidence following lung transplantation, with reported incidences from 2–33%. However, the majority of recent studies describe an AC incidence between 15–20% and an associated mortality of 2–4% (6-11). The majority of ACs occur within the first year of transplant. ACs are associated with increased mortality at 5 years post-transplant (9,12). The variability of the reported AC incidence in the literature may be due to the lack of universal definitions and grading systems for ACs. This leads to different institutional thresholds for diagnosing an AC.
Risk factors
Below we discuss the role of donor and recipient factors, surgical technique during transplant, postoperative complications, infections, and medication use on the risk of developing ACs. Although ischemia remains a major risk factor, and increases in hypoxia-inducible gene expression in donor airways have been associated with ACs, many other factors have also been shown to contribute to the development of ACs (13,14).
Donor and recipient factors
A large retrospective analysis of 16,000 lung transplant patients identified male gender, pre-transplant recipient ICU admission, and advanced recipient age as risk factors for ACs (12). However, other studies report discordant associations between age and risk of ACs (15,16). Taller recipient height and prolonged donor mechanical ventilation (50–70 hours) have also been shown to increase ACs (9). Studies have not demonstrated a consistent relationship between the development of ACs and body mass index, pre-transplant diagnosis, preoperative steroid use, cytomegalovirus status, or side of lung transplant (15,17-19).
Surgical technique
Many surgical techniques to minimize airway ischemia and reduce postoperative ACs have been explored over the years. These include “telescoping” and “end-to-end” bronchial anastomoses, vascularized pedicle tissue flaps, and bronchial artery revascularization (BAR) (20-23).
Most institutions currently prefer end-to-end anastomoses due to the increased risk of stenosis with telescoping anastomoses (20). Wrapping of the anastomosis with a vascularized flap, such as omental tissue, internal mammary pedicle, or intercostal muscle flap, was initially thought to improve anastomotic healing. However, a randomized trial did not show reduction in AC with this technique, and these are no longer routinely performed (6,24). Reducing the length of the donor bronchus also reduces the risk of ACs by minimizing the area at risk of ischemia (9,25).
Given the role of airway ischemia in the development of ACs, it would be expected that prolonged surgical ischemia time increases the risk of ACs. However, studies have not demonstrated an association between longer ischemic time and the risk of ACs. Interestingly, in bilateral sequential lung transplants, there is not an increased risk of AC at the second anastomosis (26,27).
Postoperative course
The clinical course of the recipient postoperatively may affect the subsequent risk of ACs. Patients with primary graft dysfunction have an increased incidence of ACs. Rejection episodes have been associated with greater risk of ACs, both for rejection within the first month and within three months of transplant (15,17). These findings may be due to prolonged ventilation time and exposure to high PEEP in cases of primary graft dysfunction, which may worsen airway ischemia. Likewise, acute rejection episodes are associated with a rise in pulmonary vascular resistance, which may affect blood flow to the anastomosis and thereby increase the risk of ACs.
Infections
Microbial colonization and infection in both the pre- and post-operative period have been associated with increased risk of ACs, including necrosis and stenosis. Preoperative microbial colonization with any organism is associated with increased risk of ACs; organisms implicated include Aspergillus and Haemophilus influenzae (16). Postoperative colonization with Aspergillus species, Pseudomonas aeruginosa, Pseudomonas capacia, and Actinomyces have been associated with subsequent development of ACs (15,18). Aspergillus has been linked to airway necrosis and other ACs in multiple studies, underscoring the role of aggressive antibiotic treatment in the post-transplant period to support airway healing (10,18,19).
Medications
In early lung transplant management, there was concern that corticosteroids could impair anastomotic healing and increase ACs (2). However, low to moderate doses of steroids in the preoperative and postoperative periods have not demonstrated adverse effects on airway healing (18,23,28). Corticosteroids are currently a mainstay of post-transplant triple drug immunosuppression, along with a calcineurin inhibitor and an antimetabolite.
Sirolimus is an immunosuppressant which acts by inhibiting rapamycin (mTOR). Studies on sirolimus use immediately post-transplant were ended early due to increased incidence of airway dehiscence, including fatal complications. Sirolimus use is not recommended until complete healing of the anastomosis, usually at least 90 days after transplant (29,30).
Classification of ACs
There have been several proposed systems for grading airway healing following lung transplantation. The goal of a universal grading system is to standardize definitions to reduce variability, as some institutions may consider all cases of early necrosis to be ACs, whereas others may only consider cases requiring surgery as ACs.
The Couraud system, the earliest system for grading system airway healing post lung transplant, classifies anastomoses based on the degree of mucosal necrosis. This system was found to be predictive of anastomotic complications. However, it had several pitfalls, most notably the subjective nature of grading and the lack of assessment for late ACs (31-33).
In 2014, Dutau and colleagues proposed the MDS classification system. This system assesses the macroscopic appearance of the airway (M), the airway diameter (D), and the anastomotic suture line (S). The M and D categories allow for abnormalities of the more distal airways to be described in addition to assessing the anastomosis itself (34). Most recently, ISHLT published a consensus statement building upon this work in an effort to more consistently define and grade ACs (35).
A universally adopted airway grading system is needed to accurately study ACs following lung transplant. This will enable the transplant community to assess the true incidence, morbidity and mortality of ACs.
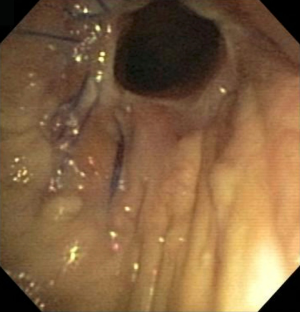
Bronchial stenosis
Bronchial stenosis is the most common AC following lung transplantation (Figure 1). The reported incidence varies from 1.6% to 32% (10,20,36,37). The etiology of bronchial stenosis is unclear. Airway inflammation, ischemia, and recurrent infection are suspected contributing factors. This can lead to ossification, calcification, and fibrovascular ingrowth of the bronchial cartilage which in turn causes stenosis (38).
While stenoses are usually at the site of surgical anastomosis, the airways distal to the anastomosis can be involved. Segmental nonanastomotic bronchial stenosis is a rare complication which can be challenging to manage (4,39). The bronchus intermedius is a frequent site of nonanastomotic stenosis. Symptomatic narrowing of the bronchus intermedius leads to vanishing bronchus intermedius syndrome (VBIS), which is associated with significant morbidity with reported overall survival of 25 months from diagnosis (40).
The diagnosis of bronchial stenosis should be suspected in transplant patients presenting with new respiratory symptoms. Common presenting symptoms include dyspnea, wheezing, cough, and recurrent pneumonia. The diagnosis may also be suspected in asymptomatic patients with reduction in spirometry or may be found during routine surveillance bronchoscopy. Pulmonary function tests may demonstrate reduced forced and peak expiratory flow, flattening of the flow-volume loop, or a biconcave flow-volume loop (41,42).
Chest imaging can be a useful tool in the diagnosis of bronchial stenosis. Chest CT with both inspiratory and expiratory images may demonstrate a fixed bronchial narrowing. In some cases, plain chest radiography can detect focal strictures. In areas of complete airway occlusion, collapse of the distal lung is seen. For example, in VBIS, chest radiography may show collapse of right lower lobe, right middle lobe, or both. Although there are many tools for assessing stenosis, flexible bronchoscopy is the gold standard for diagnosis (32,40).
Techniques used in the management of bronchial stenosis include dilation, ablation, and stenting. Treatment should be approached in a stepwise manner. A multimodal approach which combines therapies is often used.
Dilation is often the first step taken in the management of bronchial stenosis. Either rigid dilation or balloon bronchoplasty is performed. Balloon dilation is more common and has been shown to be effective at relieving dyspnea and improving expiratory lung volumes. Patients who undergo dilation may develop recurrent stenosis and require repeat balloon dilation; ultimately, they may require additional intervention such as stent placement. However, a sizable portion of patients with bronchial stenosis can be managed with bronchoplasty alone (case reports range from 26–50%) (33,43,44).
Although balloon and rigid dilation have not been compared in head to head studies, balloon bronchoplasty is generally preferred due to the ability to perform the procedure under conscious sedation, whereas rigid bronchoscopy requires general anesthesia. Additionally, since there are multiple sizes of balloons available, the balloon can be selected to best fit the stenosis and can be inflated quickly without serial upsizing. A disadvantage is that patients are unable to ventilate during inflation. Rigid bronchoscopy with dilation, on the other hand, allows for ongoing ventilation. It also enables silicone stent placement at time of dilation, if necessary.
Multiple ablation techniques are used. These include electrocautery, cryotherapy, argon plasma coagulation (APC), brachytherapy, photodynamic therapy, and neodymium-doped yttrium aluminum garnet (Nd:YAG) laser. Ablation techniques may be used in combination with balloon dilation (32,45,46).
Stenting is another treatment for bronchial stenosis which is used in cases where dilations have failed or stenosis is recurrent. Stent placement improves dyspnea and FEV1 in most patients with post-transplant bronchial stenosis. However, the risk of complications with stenting must be considered. Historically, two types of stents have been used: self-expandable metallic stents (SEMS) and silicone stents. Biodegradable (BD) stents are beginning to emerge as a new technology (47).
SEMS are associated with numerous complications in the post lung transplant population. Although frequently used in malignant airway diseases, SEMS carry high risk of complication in benign airway diseases. Early complications include migration and mucus plugging. Restenosis, bacterial colonization, and granulation tissue formation are common long-term complications. Less common is stent fracture. Notably, metallic stents are difficult to remove. The FDA in 2005 issued a black box warning regarding the placement of metallic stents in nonmalignant airway disease (48-52). SEMS have the advantage of being placed under flexible or rigid bronchoscopy, whereas silicone stents require rigid bronchoscopy and are more technically demanding. Due to the complications associated with SEMS, they are not recommended for post-transplant bronchial stenosis except as a method of last resort when other treatment options have failed.
Temporary silicone stents are now preferred in the treatment of post-transplant bronchial stenosis. Silicone stents can be safely removed in a majority of patients without recurrence of stenosis. The main complications of silicone stenting are migration, mucus plugging, and granulomas. Silicone stents are more likely than metal stents to migrate. Placement is limited to mainstream bronchi, therefore BI stenting can lead to obstruction of the right upper lobe. However, silicone stents can be customized on-site to better fit the area of stenosis (47,53-55).
New technologies are being developed for airway stenting. BD stents are made of BD polymers which hold biomechanical strength for several weeks and then degrade over the course of months. Due to their temporary nature, BD stents bypass the issue of stent removal and are less likely to become a nidus of permanent bacterial colonization. A retrospective analysis of 20 BD stents placed demonstrated safety and efficacy with 4 patients needing additional stenting for restenosis. In a prospective case series of 11 BD stents, all patients had improvement in symptoms and spirometry, and 4 patients required additional intervention due to re-stenosis (56,57).
Use of a custom 3-dimensional printed stent has also been reported (58). Custom 3-dimensional stents have the potential to uniquely fit patients with complex post-surgical airway anatomy and are a promising new technology.
Systemic and topical medication therapies have been evaluated for treatment of bronchial stenosis. Topical or submucosal treatment with mitomycin C has been used in post-transplant bronchial stenosis. Mitomycin C is an alkylating agent with anti-fibroblast activity and has been studied in the management of laryngotracheal stenosis. While there are not yet randomized controlled trials of its use in post-transplant bronchial stenosis, case studies suggest it may be beneficial (59-61). Corticosteroid injections have also been used in an attempt to reduce restenosis, although studies are lacking (62).
While sirolimus has been associated with anastomotic dehiscence early in the post-transplant course (as discussed previously), its antiproliferative properties have the potential to treat bronchial stenosis. In a retrospective review by Timofte et al. of patients with severe bronchial stenosis requiring repeat bronchoscopic intervention, 8 out of 10 patients achieved airway patency within 3 months with the addition of sirolimus to their immunosuppressive regimen (63).
Although many effective endoscopic treatments are available for bronchial stenosis, refractory cases which fail to respond to the above treatments should be evaluated by a multidisciplinary team and considered for surgical management. Sleeve resection is a reported treatment for VBIS. Bronchoplasty, bronchial anastomosis reconstruction, lobectomy, pneumonectomy, and retransplantation have also been described (37,64).
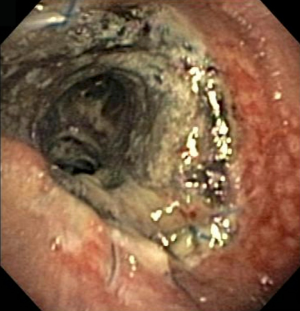
Necrosis and dehiscence
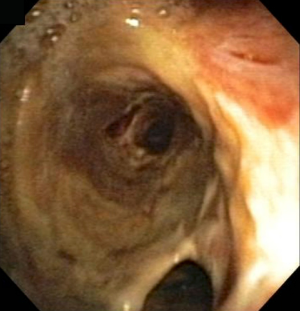
Nearly all transplanted lungs experience some degree of airway necrosis as a result of ischemic injury (Figure 2). This necrosis usually heals by the sixth week post-transplantation. However, some cases of necrosis progress to dehiscence. Necrosis can be conceptualized as a spectrum encompassing normal healing with superficial mucosal slough, severe necrosis with full thickness or circumferential involvement, and frank airway dehiscence (Figure 3) (36).
Airway dehiscence is a severe complication which carries a 3- to 5-fold increased risk of 1-year mortality (65). Although catastrophic, dehiscence is rare, with a reported rate of 1–10%. Factors which may increase the risk of dehiscence include Aspergillus colonization and prolonged positive pressure ventilation (66).
Dehiscence may occur even in patients with an uncomplicated operative and postoperative course. It may present in the weeks following transplant with sepsis, failure to wean from ventilator, pneumothorax or pneumomediastinum, or persistent air leak. The mainstay of diagnosis is a high index of suspicion and close inspection of the anastomosis on surveillance bronchoscopy. Chest radiography may identify secondary findings such as pneumomediastinum, but it is not a reliable method of diagnosing dehiscence. Chest CT has demonstrated excellent sensitivity and specificity for detecting dehiscence. However, as with most ACs, bronchoscopy remains the gold standard for diagnosis. Unlike CT imaging, bronchoscopy is able to detect early mucosal necrosis, enabling providers to identify airways at risk of progression to dehiscence (4,67). In cases of frank dehiscence, unraveling of sutures at the anastomosis may also be seen on bronchoscopy (66).
Management of necrosis and dehiscence depends on the severity of the lesion. Mild cases of mucosal slough without bronchial wall necrosis may be managed with mechanical debulking and antibiotic treatment. More severe cases often require bronchoscopic or surgical intervention. The data on bronchoscopic topical interventions is lacking. Limited cases describe the use of alpha-cyanoacrylate glue or autologous platelet-derived wound healing factor (27,68,69). Surgical techniques in the management of bronchial dehiscence include reanastomosis, flap bronchoplasty, and retransplantation (27,70).
Uncovered metallic stents (SEMS) can be used in cases of dehiscence to stent over the defect and allow time for tissue healing. In this case, the previously described propensity for granulation tissue formation at site of stent placement works in the patient’s favor by promoting healing of the dehiscence. The area is then monitored for epithelialization, and the stent is removed when epithelialization occurs. SEMS placement in airway dehiscence may be technically challenging due to the need for precise placement. After stent removal, patients will need bronchoscopic surveillance for the development of further complications such as bronchial stenosis. Silicone stents are not used in the management of dehiscence due to the risk of migration and the force required to insert a silicone stent in an already fragile area (71).
Dehiscence remains a devastating AC, and early diagnosis and treatment of airway necrosis is key to preventing this dreaded condition.
Fistula
Bronchial fistulae are a rare but serious complication of lung transplantation and result in an abnormal connection between the airway and the mediastinum, pleural space, or vasculature. In immunocompromised populations such as lung transplant patients, endobronchial fungal infections, especially Aspergillus, increase risk of formation of all types of fistulae (72-75). Fistula may present as dyspnea, sepsis, pneumothorax, subcutaneous emphysema, or a persistent air leak typically in the setting of dehiscence. Management is similar to that of anastomotic dehiscence. Success depends on the location and size of the defect.
Bronchovascular fistulae after lung transplantation are limited to case reports. Fistula formation has been associated with necrotizing pneumonia and with erosion of bronchial stents into nearby vascular structures. Bronchovascular fistulae are often life-threatening, and massive hemoptysis may be the presenting symptom. Endovascular stenting and surgical treatment with pneumonectomy or fistula resection and reconstruction have been described, although these carry extremely high morbidity (76-78).
Anastomotic infections
Infection is the most common complication of lung transplant. Lung transplant patients are at increased risk of infection due to many factors, including immunosuppression, impaired cough and airway clearance, altered phagocyte function, and decreased lymphatic drainage. Pre-transplant colonization is also common (79).
Infections at the anastomosis carry an incidence of 5% in lung transplant recipients. More importantly, they are often the precursor to the other complications discussed (80,81). Diagnosis usually occurs at bronchoscopy. Inflammation, ulceration, or pseudomembranes are often seen along the airway and are treated with debridement and antibiotics. Protocols vary by institution and include local debridement as well as systemic and inhaled regimens with voriconazole, itraconazole, and inhaled amphotericin commonly used.
Excessive granulation tissue
Hyperplastic granulation tissue can cause endoluminal obstruction of the airway in post-lung transplant patients. It has been reported in 6–20% of transplant recipients and usually develops several months post-transplant. The anastomotic site is commonly involved (27,82,83). The etiology of this excessive granulation tissue proliferation is thought to be related to an inflammatory response to airway injury. This process has been compared to keloid formation (84,85).
Granulation tissue is a common complication of stent placement, with estimated incidence of 12–36%, although one retrospective analysis of silicone stents reported granuloma formation in over 50% of stents (4,47,86). Aspergillus colonization has also been associated with the formation of exuberant granulation tissue (87).
The symptoms of obstructive granulation tissue may include dyspnea, cough, post-obstructive pneumonia, hemoptysis, and difficulty clearing secretions. Reduction in spirometry may be seen depending on the severity of the obstruction. Bronchoscopy with visualization of granulation is the gold standard for diagnosis (4).
Management depends on the severity of the obstruction. Many obstructions recur and require serial endoscopic interventions. Small non-obstructing lesions may be debulked with forceps using flexible bronchoscopy or using the beveled edge of a rigid bronchoscope. More severe obstructions can be treated with cryotherapy, cryodebridement, high-dose-rate endobronchial brachytherapy (HDR-EB), Nd:YAG laser ablation, or argon plasma coagulation (46).
Hot or cold modalities as well as the microdebrider can be used to restore patency. A superior safety profile, the cryosensitivity of granulation tissue, excellent hemostasis, and the ability to use around stents without the risk of ignition even in high concentrations of oxygen make cryotherapy an appealing option. The full effect of cryotherapy treatment may not be reached until 8–10 days after treatment. APC, electrocautery, and laser ablation have a long history of successful management (83,88-90). HDR-EB or photodynamic therapy have also been reported but should be used with extreme caution as serious complications, including fatal hemoptysis, have been described (82,91,92).
Topical application of mitomycin C to prevent the recurrence of granulation tissue after intervention has been described (93,94). Although randomized trials in this area are needed, this agent is often used due to its favorable safety profile. Intralesional steroids such as triamcinolone have not been well studied in this area.
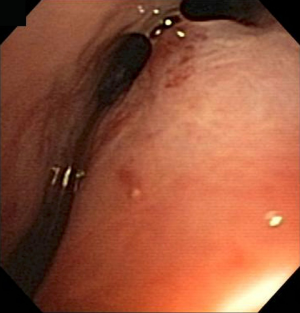
Tracheobronchomalacia
Bronchomalacia is characterized by dynamic airway collapse. In contrast to bronchial stenosis or obstructive granulation, which cause fixed obstruction, bronchomalacia is obstruction of the airways during expiration (Figure 4). This is attributed to weakness of the airway cartilage. Malacia is defined as airway narrowing of >50% during expiration. Patients may have combined lesions with both stenosis and superimposed malacia (33,95,96).
The symptoms of tracheobronchomalacia are nonspecific and similar to symptoms for other ACs: dyspnea, cough, inability to clear secretions, recurrent pneumonia, and hemoptysis. Patients may have a “barking” or seal-like cough, which is unique to tracheobronchomalacia. Spirometry usually demonstrates an obstructive pattern with low forced expiratory volume at one second and low peak expiratory flow rates. The obstruction is usually more pronounced during expiration, and variable obstruction and expiratory oscillations can be seen on flow-volume loops (97).
The gold standard for diagnosis of tracheobronchomalacia is direct visualization of dynamic airway collapse with bronchoscopy. CT axial imaging with forced expiratory images may demonstrate dynamic airway narrowing (95,96).
The principles of management of post-transplant tracheobronchomalacia are derived from management of non-transplant populations. These principles include pulmonary hygiene, inhaled mucolytics, and noninvasive positive pressure ventilation (4,96).
If severe symptoms are present, stenting is another treatment option (98). Due to the risk of complications with stenting, close follow up with is required. Silicone stents are preferred (4,99). Stents placed for bronchomalacia have been associated with improvement in FEV1 and symptoms of dyspnea (33,98). If there is no improvement in symptoms with stenting, removal of the stent is recommended (96). If there is symptomatic improvement, we recommend trial of silicone stent removal at 6 to 12 months. Due to the long-term complications associated with stents, these should be reserved for patients who have severe impairment despite medical management.
BAR
Current lung transplant techniques disrupt the bronchial artery circulation and leave the transplant dependent on retrograde blood flow from the pulmonary circulation as described above. BAR at the time of transplant creates an anastomosis between the donor and recipient bronchial artery. BAR is an appealing prospect as it has the potential to decrease subsequent ACs by improving blood flow to the graft.
A pilot study of 131 patients from two centers demonstrated 90% success rate of BAR. In cases where the BAR was successful, patients had normal airway healing. The success rate of BAR was higher for bilateral lung transplants than single lung transplants. Double lung transplant patients who underwent BAR, irrespective of success of revascularization, had superior 5- and 10-year mortality compared to ISHLT aggregate data (13). Despite these potential advantages, BAR has several downsides. It confers additional risks of bleeding and requires careful dissection of the donor lung. It also carries risks of prolonged cardiopulmonary bypass time and prolonged graft ischemic time. It is not routinely performed in lung transplantation, although further multicenter studies are needed to assess the risks of BAR as well as the potential for improved airway healing.
Summary
ACs following lung transplantation remain a major source of post-transplant morbidity and mortality. These complications can negatively impact quality of life and increase the need for office visits, serial procedures, and hospitalizations. The incidence of ACs has decreased in the decades since the advent of lung transplantation with advances in surgical technique, medical management, and immunosuppression. When ACs do occur, there are a variety of treatment modalities available, particularly bronchoscopic treatments. Management of ACs is best undertaken by a multidisciplinary team at a transplant center with experience in this area.
Further studies into management strategies are needed. There is not yet randomized control trial data to support the superiority of any one management approach. A universal grading system to identify and describe ACs is an important step in better understanding ACs in this complex patient population.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Douglas Kyle Hogarth and Jonathan S. Kurman) for the series “Interventional Pulmonology and Advanced Bronchoscopy” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.12.02). The series “Interventional Pulmonology and Advanced Bronchoscopy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nelems JM, Rebuck AS, Cooper JD, et al. Human Lung Transplantation. Chest 1980;78:569-73. [Crossref] [PubMed]
- Toronto Lung Transplant Group. Unilateral lung transplantation for pulmonary fibrosis. N Engl J Med 1986;314:1140-5. [Crossref] [PubMed]
- Wildevuur CR, Benfield JR. A review of 23 human lung transplantations by 20 surgeons. Ann Thorac Surg 1970;9:489-515. [Crossref] [PubMed]
- Santacruz JF, Mehta AC. Airway Complications and Management after Lung Transplantation. Proc Am Thorac Soc 2009;6:79-93. [Crossref] [PubMed]
- Frye L, Machuzak M. Airway Complications After Lung Transplantation. Clin Chest Med 2017;38:693-706. [Crossref] [PubMed]
- Schmid RA, Boehler A, Speich R, et al. Bronchial anastomotic complications following lung transplantation: Still a major cause of morbidity? Eur Respir J 1997;10:2872-5. [Crossref] [PubMed]
- Murthy SC, Blackstone EH, Gildea TR, et al. Impact of Anastomotic Airway Complications After Lung Transplantation. Ann Thorac Surg 2007;84:401-9, 409.e1-4.
- Murthy SC, Gildea TR, Machuzak MS. Anastomotic airway complications after lung transplantation. Curr Opin Organ Transplant 2010;15:582. [Crossref] [PubMed]
- Van De Wauwer C, Van Raemdonck D, Verleden GM, et al. Risk factors for airway complications within the first year after lung transplantation. Eur J Cardiothorac Surg 2007;31:703-10. [Crossref] [PubMed]
- Herrera JM, McNeil KD, Higgins RSD, et al. Airway complications after lung transplantation: Treatment and long-term outcome. Ann Thorac Surg 2001;71:989-93. [Crossref] [PubMed]
- Alvarez A, Algar J, Santos F, et al. Airway complications after lung transplantation: A review of 151 anastomoses. Eur J Cardiothorac Surg 2001;19:381-7. [Crossref] [PubMed]
- Awori Hayanga JW, Aboagye JK, Shigemura N, et al. Airway complications after lung transplantation: Contemporary survival and outcomes. J Heart Lung Transplant 2016;35:1206-11. [Crossref] [PubMed]
- Pettersson GB, Yun JJ, Nørgaard MA. Bronchial artery revascularization in lung transplantation: Techniques, experience, and outcomes. Curr Opin Organ Transplant 2010;15:572. [Crossref] [PubMed]
- Kraft BD, Suliman HB, Colman EC, et al. Hypoxic Gene Expression of Donor Bronchi Linked to Airway Complications after Lung Transplantation. Am J Respir Crit Care Med 2016;193:552-60. [Crossref] [PubMed]
- Thistlethwaite PA, Yung G, Kemp A, et al. Airway stenoses after lung transplantation: Incidence, management, and outcome. J Thorac Cardiovasc Surg 2008;136:1569-75. [Crossref] [PubMed]
- Yserbyt J, Dooms C, Vos R, et al. Anastomotic airway complications after lung transplantation: Risk factors, treatment modalities and outcome-a single-centre experience. Eur J Cardiothorac Surg 2016;49:e1-8. [Crossref] [PubMed]
- Ruttmann E, Ulmer H, Marchese M, et al. Evaluation of factors damaging the bronchial wall in lung transplantation. J Heart Lung Transplant 2005;24:275-81. [Crossref] [PubMed]
- Moreno P, Alvarez A, Algar FJ, et al. Incidence, management and clinical outcomes of patients with airway complications following lung transplantation. Eur J Cardiothorac Surg 2008;34:1198-205. [Crossref] [PubMed]
- Weder W, Inci I, Korom S, et al. Airway complications after lung transplantation: Risk factors, prevention and outcome. Eur J Cardiothorac Surg 2009;35:293-8. [Crossref] [PubMed]
- Garfein ES, McGregor CC, Galantowicz ME, et al. Deleterious effects of telescoped bronchial anastomosis in single and bilateral lung transplantation. Ann Transplant 2000;5:5-11. [PubMed]
- Schröder C, Scholl F, Daon E, et al. A modified bronchial anastomosis technique for lung transplantation. Ann Thorac Surg 2003;75:1697-704. [Crossref] [PubMed]
- Morgan E, Lima O, Goldberg M, et al. Successful revascularization of totally ischemic bronchial autografts with omental pedicle flaps in dogs. J Thorac Cardiovasc Surg 1982;84:204-10. [Crossref] [PubMed]
- Miller JD, DeHoyos A. An evaluation of the role of omentopexy and of early perioperative corticosteroid administration in clinical lung transplantation. The University of Toronto and Washington University Lung Transplant Programs. J Thorac Cardiovasc Surg 1993;105:247-52. [Crossref] [PubMed]
- Khaghani A, Tadjkarimi S, al-Kattan K, et al. Wrapping the anastomosis with omentum or an internal mammary artery pedicle does not improve bronchial healing after single lung transplantation: Results of a randomized clinical trial. J Heart Lung Transplant 1994;13:767-73. [PubMed]
- van Berkel V, Guthrie TJ, Puri V, et al. Impact of Anastomotic Techniques on Airway Complications After Lung Transplant. Ann Thorac Surg 2011;92:316-20; discussion 320-1. [Crossref] [PubMed]
- Colquhoun IW, Gascoigne AD, Au J, et al. Airway complications after pulmonary transplantation. Ann Thorac Surg 1994;57:141-5. [Crossref] [PubMed]
- Kshettry VR, Kroshus TJ, Hertz MI, et al. Early and Late Airway Complications After Lung Transplantation: Incidence and Management. Ann Thorac Surg 1997;63:1576-83. [Crossref] [PubMed]
- Park SJ, Nguyen DQ, Savik K, et al. Pre-transplant corticosteroid use and outcome in lung transplantation. J Heart Lung Transplant 2001;20:304-9. [Crossref] [PubMed]
- King-Biggs MB, Dunitz J, Park S, et al. Airway anastomotic dehiscence associated with use of sirolimus immediately after lung transplantation. Transplantation 2003;75:1437-43. [Crossref] [PubMed]
- Groetzner J, Kur F, Spelsberg F, et al. Airway anastomosis complications in de novo lung transplantation with sirolimus-based immunosuppression. J Heart Lung Transplant 2004;23:632-8. [Crossref] [PubMed]
- Couraud L, Nashef SA, Nicolini P, et al. Classification of airway anastomotic healing. Eur J Cardiothorac Surg 1992;6:496-7. [Crossref] [PubMed]
- Shennib H, Massard G. Airway complications in lung transplantation. Ann Thorac Surg 1994;57:506-11. [Crossref] [PubMed]
- Chhajed PN, Malouf MA, Tamm M, et al. Interventional Bronchoscopy for the Management of Airway Complications Following Lung Transplantation. Chest 2001;120:1894-9. [Crossref] [PubMed]
- Dutau H, Vandemoortele T, Laroumagne S, et al. A new endoscopic standardized grading system for macroscopic central airway complications following lung transplantation: The MDS classification. Eur J Cardiothorac Surg 2014;45:e33-8. [Crossref] [PubMed]
- Crespo MM, McCarthy DP, Hopkins PM, et al. ISHLT Consensus Statement on adult and pediatric airway complications after lung transplantation: Definitions, grading system, and therapeutics. J Heart Lung Transplant 2018;37:548-63. [Crossref] [PubMed]
- Wilson IC, Hasan A, Healey M, et al. Healing of the bronchus in pulmonary transplantation. Eur J Cardiothorac Surg 1996;10:521-6; discussion 526-7. [Crossref] [PubMed]
- Marulli G, Loy M, Rizzardi G, et al. Surgical treatment of posttransplant bronchial stenoses: Case reports. Transplant Proc 2007;39:1973-5. [Crossref] [PubMed]
- Yousem SA, Dauber JH, Griffith BR. Bronchial Cartilage Alterations in Lung Transplantation. Chest 1990;98:1121-4. [Crossref] [PubMed]
- Hasegawa T, Iacono AT, Orons PD, et al. Segmental nonanastomotic bronchial stenosis after lung transplantation. Ann Thorac Surg 2000;69:1020-4. [Crossref] [PubMed]
- Shah SS, Karnak D, Minai O, et al. Symptomatic Narrowing or Atresia of Bronchus Intermedius Following Lung Transplantation Vanishing Bronchus Intermedius Syndrome (VBIS). Chest 2006;130:236S. [Crossref]
- Anzueto A, Levine SM, Tillis WP, et al. Use of the Flow-Volume Loop in the Diagnosis of Bronchial Stenosis After Single Lung Transplantation. Chest 1994;105:934-6. [Crossref] [PubMed]
- Neagos GR, Martinez FJ, Deeb GM, et al. Diagnosis of Unilateral Mainstem Bronchial Obstruction Following Single-Lung Transplantation With Routine Spirometry. Chest 1993;103:1255-8. [Crossref] [PubMed]
- De Gracia J, Culebras M, Alvarez A, et al. Bronchoscopic balloon dilatation in the management of bronchial stenosis following lung transplantation. Respir Med 2007;101:27-33. [Crossref] [PubMed]
- Mayse ML, Greenheck J, Friedman M, et al. Successful Bronchoscopic Balloon Dilation of Nonmalignant Tracheobronchial Obstruction Without Fluoroscopy. Chest 2004;126:634-7. [Crossref] [PubMed]
- Colt HG, Janssen JP, Dumon JF, et al. Endoscopic Management of Bronchial Stenosis after Double Lung Transplantation. Chest 1992;102:10-6. [Crossref] [PubMed]
- Mathur PN, Wolf KM, Busk MF, et al. Fiberoptic Bronchoscopic Cryotherapy in the Management of Tracheobronchial Obstruction. Chest 1996;110:718-23. [Crossref] [PubMed]
- Dutau H, Cavailles A, Sakr L, et al. A retrospective study of silicone stent placement for management of anastomotic airway complications in lung transplant recipients: Short- and long-term outcomes. J Heart Lung Transplant 2010;29:658-64. [Crossref] [PubMed]
- Gottlieb J, Fuehner T, Dierich M, et al. Are metallic stents really safe? A long-term analysis in lung transplant recipients. Eur Respir J 2009;34:1417-22. [Crossref] [PubMed]
- Gaissert HA, Grillo HC, Wright CD, et al. Complication of benign tracheobronchial strictures by self-expanding metal stents. J Thorac Cardiovasc Surg 2003;126:744-7. [Crossref] [PubMed]
- Kapoor BS, May B, Panu N, et al. Endobronchial Stent Placement for the Management of Airway Complications after Lung Transplantation. J Vasc Interv Radiol 2007;18:629-32. [Crossref] [PubMed]
- Dutau H. Editorial comment. Airway stenting for benign tracheal stenosis: What is really behind the choice of the stent? Eur J Cardiothorac Surg 2011;40:924-5. [PubMed]
- Madden BP, Loke TK, Sheth AC. Do Expandable Metallic Airway Stents Have a Role in the Management of Patients With Benign Tracheobronchial Disease? Ann Thorac Surg 2006;82:274-8. [Crossref] [PubMed]
- Wood DE, Liu YH, Vallières E, et al. Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg 2003;76:167-72; discussion 173-4. [Crossref] [PubMed]
- Breen DP, Dutau H. On-Site Customization of Silicone Stents: Towards Optimal Palliation of Complex Airway Conditions. Respiration 2009;77:447-53. [Crossref] [PubMed]
- Fernandez-Bussy S, Akindipe O, Kulkarni V, et al. Clinical Experience With a New Removable Tracheobronchial Stent in the Management of Airway Complications After Lung Transplantation. J Heart Lung Transplant 2009;28:683-8. [Crossref] [PubMed]
- Lischke R, Pozniak J, Vondrys D, et al. Novel biodegradable stents in the treatment of bronchial stenosis after lung transplantation. Eur J Cardiothorac Surg 2011;40:619-624. [PubMed]
- Fuehner T, Suhling H, Greer M, et al. Biodegradable stents after lung transplantation. Transpl Int 2013;26:e58-60. [Crossref] [PubMed]
- Guibert N, Didier A, Moreno B, et al. Treatment of Post-transplant Complex Airway Stenosis with a Three-Dimensional, Computer-assisted Customized Airway Stent. Am J Respir Crit Care Med 2017;195:e31-3. [Crossref] [PubMed]
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: Are two applications better than one? Laryngoscope 2009;119:272-83. [Crossref] [PubMed]
- Cosano-Povedano J, Muñoz-Cabrera L, Jurado-Gámez B, et al. Topical Mitomycin C for Recurrent Bronchial Stenosis After Lung Transplantation: A Report of 2 Cases. J Bronchol Interv Pulmonol 2008;15:281-3.
- Davidson K, Elmasri M, Wahidi M, et al. Management of Lung Transplant Bronchial Stenosis With Mitomycin C. J Bronchol Interv Pulmonol 2019;26:124-8.
- Tremblay A, Coulter TD, Mehta AC. Modification of a Mucosal-Sparing Technique Using Electrocautery and Balloon Dilatation in the Endoscopic Management of Web-Like Benign Airway Stenosis J Bronchol Interv Pulmonol 2003;10:268-71.
- Timofte I, Crespo M, Bermudez C, et al. Addition of Rapamycin to the Immunosuppressant Regimen Improves Airway Patency in Lung Transplant Patients With Refractory Bronchial Stenosis. Chest 2013;144:1014A. [Crossref]
- Souilamas R, Wermert D, Guillemain R, et al. Uncommon Combined Treatment of Nonanastomotic Bronchial Stenosis After Lung Transplantation. J Bronchol Interv Pulmonol 2008;15:54.
- Mirza O, Garcia H, Mohanka M, et al. Independent Predictors of Anastomotic Dehiscence and Its Association With Survival Among Patients With Lung Transplantation: An Analysis of UNOS Database. Chest 2017;152:A1102. [Crossref]
- Usuda K, Gildea TR, Pandya C, et al. Bronchial Dehiscence. J Bronchol Interv Pulmonol 2005;12:164.
- Herman SJ, Weisbrod G, Weisbrod L, et al. Chest radiographic findings after bilateral lung transplantation. AJR Am J Roentgenol 1989;153:1181-5. [Crossref] [PubMed]
- Maloney JD, Weigel TL, Love RB. Endoscopic repair of bronchial dehiscence after lung transplantation. Ann Thorac Surg 2001;72:2109-11. [Crossref] [PubMed]
- Chang CC, Hsu HH, Kuo SW, et al. Bronchoscopic gluing for post-lung-transplant bronchopleural fistula. Eur J Cardiothorac Surg 2007;31:328-30. [Crossref] [PubMed]
- Schäfers HJ, Schäfer CM, Zink C, et al. Surgical treatment of airway complications after lung transplantation. J Thorac Cardiovasc Surg 1994;107:1476-80. [Crossref] [PubMed]
- Mughal MM, Gildea TR, Murthy S, et al. Short-Term Deployment of Self-Expanding Metallic Stents Facilitates Healing of Bronchial Dehiscence. Am J Respir Crit Care Med 2005;172:768-71. [Crossref] [PubMed]
- Mehrad B, Paciocco G, Martinez FJ, et al. Spectrum of Aspergillus Infection in Lung Transplant Recipients. Chest 2001;119:169-75. [Crossref] [PubMed]
- Karnak D, Avery RK, Gildea TR, et al. Endobronchial Fungal Disease: An Under-Recognized Entity. Respiration 2007;74:88-104. [Crossref] [PubMed]
- Argento AC, Wolfe CR, Wahidi MM, et al. Bronchomediastinal Fistula Caused by Endobronchial Aspergilloma. Ann Am Thorac Soc 2015;12:91-5. [Crossref] [PubMed]
- Ahuja J, Kanne JP. Thoracic Infections in Immunocompromised Patients. Radiol Clin North Am 2014;52:121-36. [Crossref] [PubMed]
- Samano MN, Saka JA, Caramori ML, et al. Fatal bronchovascular fistula after a single lung transplantation: A case report. Clinics 2009;64:1031-3. [Crossref] [PubMed]
- Sourrouille I, Mordant P, Karsenti A, et al. Successful Surgical Treatment of a Posttransplantation Bronchovascular Fistula Involving the Pulmonary Vein. Ann Thorac Surg 2011;92:1891-3. [Crossref] [PubMed]
- Knight J, Elwing JM, Milstone A. Bronchovascular Fistula Formation: A Rare Airway Complication After Lung Transplantation. J Heart Lung Transplant 2008;27:1179-85. [Crossref] [PubMed]
- Felton TW, Roberts SA, Isalska B, et al. Isolation of Aspergillus species from the airway of lung transplant recipients is associated with excess mortality. J Infect 2012;65:350-6. [Crossref] [PubMed]
- Hadjiliadis D, Howell DN, Davis RD, et al. Anastomotic infections in lung transplant recipients. Ann Transplant 2000;5:13-9. [PubMed]
- Parada MT, Alba A, Sepúlveda C. Early and Late Infections in Lung Transplantation Patients. Transplant Proc 2010;42:333-5. [Crossref] [PubMed]
- Tendulkar RD, Fleming PA, Reddy CA, et al. High-Dose-Rate Endobronchial Brachytherapy for Recurrent Airway Obstruction From Hyperplastic Granulation Tissue. Int J Radiat Oncol Biol Phys 2008;70:701-6. [Crossref] [PubMed]
- Maiwand MO. The role of cryotherapy for airway complications after lung and heart-lung transplantation. Eur J Cardiothorac Surg 1997;12:549-54. [Crossref] [PubMed]
- Kennedy AS, Sonett JR, Orens JB, et al. High dose rate brachytherapy to prevent recurrent benign hyperplasia in lung transplant bronchi: Theoretical and clinical considerations. J Heart Lung Transplant 2000;19:155-9. [Crossref] [PubMed]
- Yousem SA, Duncan SR, Griffith BP. Interstitial and airspace granulation tissue reactions in lung transplant recipients. Am J Surg Pathol 1992;16:877-84. [Crossref] [PubMed]
- Saad CP, Murthy S, Krizmanich G, et al. Self-Expandable Metallic Airway Stents and Flexible Bronchoscopy: Long-term Outcomes Analysis. Chest 2003;124:1993-9. [Crossref] [PubMed]
- Nathan SD, Shorr AF, Schmidt ME, et al. Aspergillus and Endobronchial Abnormalities in Lung Transplant Recipients. Chest 2000;118:403-7. [Crossref] [PubMed]
- Yılmaz A, Aktaş Z, Alici İO, et al. Cryorecanalization: Keys to success. Surg Endosc 2012;26:2969-74. [Crossref] [PubMed]
- Keller CA, Hinerman R, Singh A, et al. The Use of Endoscopic Argon Plasma Coagulation in Airway Complications After Solid Organ Transplantation. Chest 2001;119:1968-75. [Crossref] [PubMed]
- Madden BP. Successful resection of obstructing airway granulation tissue following lung transplantation using endobronchial laser (Nd:YAG) therapy. Eur J Cardiothorac Surg 1997;12:480-5. [Crossref] [PubMed]
- Halkos ME, Godette KD, Lawrence EC, et al. High Dose Rate Brachytherapy in the Management of Lung Transplant Airway Stenosis. Ann Thorac Surg 2003;76:381-4. [Crossref] [PubMed]
- Hara R, Itami J, Aruga T, et al. Risk factors for massive hemoptysis after endobronchial brachytherapy in patients with tracheobronchial malignancies. Cancer 2001;92:2623-7. [Crossref] [PubMed]
- Erard AC, Monnier P, Spiliopoulos A, et al. Mitomycin C for Control of Recurrent Bronchial Stenosis. Chest 2001;120:2103-5. [Crossref] [PubMed]
- Penafiel A, Lee P, Hsu A, et al. Topical Mitomycin-C for Obstructing Endobronchial Granuloma. Ann Thorac Surg 2006;82:e22-3. [Crossref] [PubMed]
- Ridge CA, O’Donnell CR, Lee EY, et al. Tracheobronchomalacia: Current Concepts and Controversies. J Thorac Imaging 2011;26:278. [Crossref] [PubMed]
- Carden KA, Ernst A. Management of Tracheobronchomalacia. In: Thoracic Endoscopy: Advances in Interventional Pulmonology. 2008:344-51.
- Majid A, Sosa AF, Ernst A, et al. Pulmonary Function and Flow-Volume Loop Patterns in Patients with Tracheobronchomalacia. Respir Care 2013;58:1521-6. [Crossref] [PubMed]
- Susanto I, Peters JI, Levine SM, et al. Use of Balloon-Expandable Metallic Stents in the Management of Bronchial Stenosis and Bronchomalacia After Lung Transplantation. Chest 1998;114:1330-5. [Crossref] [PubMed]
- Mahajan AK, Folch E, Khandhar SJ, et al. The Diagnosis and Management of Airway Complications Following Lung Transplantation. Chest 2017;152:627-38. [Crossref] [PubMed]
Cite this article as: Frye L, Phillips EK. Management of airway complications following lung transplantation. Shanghai Chest 2020;4:28.