
The role of endobronchial ultrasound transbronchial needle aspiration in patients candidate to pneumonectomy
Introduction
Lung cancer remains the leading cause of death accounting for 14% of all cancer-related deaths in 2018 (1). Among the different histotypes, non-small-cell lung cancer (NSCLC) is the most frequent lung cancer-related death with an overall 5-year survival rate ranging from 60% for early-stage disease to 36% in advanced stages (IIIA) (2,3).
Surgery is the mainstay of the treatment of lung cancer and complete resection with radical lymph node removal is the best treatment choice. For tumors with large dimensions, located in the central area of the lungs or invading other structures such as the carina, a major surgical resection like pneumonectomy may be required. Considering the high rate of morbidity and mortality associated with pneumonectomy (4), careful patient staging is pivotal to determine appropriate surgery feasibility, resection probability and to evaluate any neo-adjuvant treatment (5).
Pre-operative assessment of patients’ candidates to pneumonectomy includes a non-invasive imaging mediastinal staging with computed tomography (CT) and 18-fluorodeoxyglucose positron emission tomography (PET) and pre-operative cell typing of the tumor.
Mediastinal non-invasive staging is associated with low accuracy and a high false-positive rates of 42% and 37% for CT and PET scans respectively (5) and so invasive mediastinal staging is mandatory to better stratify the correct disease stage.
Mediastinal lymph node staging was traditionally performed with mediastinoscopy, but the use of the procedure has progressively declined, due to its high invasiveness and risk of complications (6). In the last years endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) demonstrated a high accuracy in the diagnosis of lung cancer and become crucial in clinical practice as the standard care for the diagnosis and staging of NSCLC (7).
For mediastinal staging, EBUS-TBNA is the best diagnostic and staging tool with an accuracy of 80–90%, reaching 93% in clinical N2 suspected patients, with almost no complications (7-9).
Objectives
The aim of the study was to evaluate the role of EBUS-TBNA is the management of patient’s candidate to pneumonectomy evaluating the sensibility, negative predictive value and diagnostic accuracy of EBUS-TBNA lymph nodes sampling in this cohort of patients.
Methods
We retrospectively reviewed the medical records of 230 consecutive patient candidates for pneumonectomy at our Institution from January 2012 to December 2019.
The Ethics Committee approved data collection and analysis and waived the need for written consent.
Variables extracted from our database included: demographic characteristics (age, sex), clinical information, pre-operative tests (cardiac-pulmonary tests, CT, PET), mediastinal staging information, clinical staging according to the TNM 8th edition, histopathologic diagnosis, neoadjuvant treatment, surgical information, pathological TNM, recurrence and follow-up.
EBUS-TBNA was performed as already published (10). Briefly, the procedure was performed under local anesthesia (1% lidocaine) and moderate sedation provided by an anesthesiologist with spontaneous ventilation. All EBUS-TBNA procedures were performed using a convex-probe (EBUS Convex Probe BF-UC180F; Olympus) and a dedicated ultrasound processor (EU-ME1 or 2; Olympus). EBUS-TBNA specimens were collected with a 22-gauge dedicated needle (Vizishot NA-201SX-4022; Olympus). A very small amount of the aspirated material was smeared onto glass slides for immediate rapid on-site evaluation (ROSE) and evaluated by the pathologist to confirm adequate lymph node or tumor cell material. The remaining sample were fixed in a formalin-like solution for later histological evaluation.
Results
During the study period, 230 patients were candidates for pneumonectomy at our Institution.
The final analysis included 80 patients (60 males, 75%) with a median age of 70 (range, 24–92) years.
Twenty-five patients (31.3%) presented comorbidities: 19 (23.8%) cardiac comorbidities, 2 (2.5%) pulmonary comorbidities and 4 patients (5%) had both. Five patients (6.3%) had a history of previous malignancies (breast, ovary, colon or bladder cancer).
Cardiac-pulmonary tests showed a median FEV1 of 86.1% and DLCO of 84.0%. Clinical-demographic characteristics are reported in Table 1.
Table 1
| Characteristics | N of patients (% of 80) |
|---|---|
| Gender | |
| Male | 60 (75.0) |
| Female | 20 (25.0) |
| Age [range], years | 70 [24–92] |
| Comorbidity | |
| Cardiac | 19 (23.8) |
| Pulmonary | 2 (2.5) |
| Cardiac-pulmonary | 4 (5.0) |
| Previous malignancy | 5 (6.3) |
The non-invasive mediastinal staging with CT and PET scans showed a suspect mediastinal lymph node involvement (cN2) in 48 patients (60%). Twenty-one patients (26.3%) showed suspicious cN2 at CT not confirmed at PET scan and 11 (13.8%) patients presented a PET pathological uptake without a corresponding imaging at CT scan.
EBUS-TBNA was performed to achieve both cell typing and lymph node staging in 49 patients (61.3%); for cN2 sampling after a previous diagnosis of the primary tumor in 14 (17.5%) patients; to evaluate endobronchial involvement and to evaluate a cN2 suspicion in 5 (6.3%) cases; to exclude a cN3 suspicious in 6 (7.5%) and also for mutations assessment status in 6 patients (7.5%).
At non-invasive staging, 26 patients (32.5%) where staged as cIIIA, 52 (65%) were cIIIB (due to tumor dimension or tumor invasion of adjacent region) and 2 patients (2.5%) were considered cIIIB for suspected contralateral lymph node metastasis.
After EBUS-TBNA procedure, 14 patients cIIIA patients were confirmed as pIIIA, 11 were downstaged as pIIB and one as pIIA. Fourteen patients with clinical IIIB (cT3 or cT4 stages), were confirmed as pIIIB, 19 were downstaged as pIIIA and 19 pIIB. Between the 2 patients staged as cIIIB for cN3 disease, one was confirmed as stage pIIIB and the other resulted pN0 after EBUS-TBNA.
A total of 109 lymph node stations were biopsied, with 136 lymph nodes sampled. The most frequent station sampled was subcarinal (station #7, 48.6%), followed by the right lower paratracheal station (#4R, 24.8%) or both (21.1%).
Tumor cell type were: 43 adenocarcinomas (53.8%), 29 (36.3%) squamous cell carcinomas, 1 (1.3%) adenosquamous carcinoma and 6 (7.5%) cases of poor differentiated NSCLC.
The results of EBUS-TBNA showed a sensibility, negative predictive value and diagnostic accuracy of 86.7%, 94.4% and 95.9% respectively.
In two patients, EBUS-TBNA was negative for malignancy in subcarinal stations (#7) and resulted a pN2 after surgery, due to station #5 involvement. Those patients were considered true negative cases at the statistical analysis since that EBUS-TBNA can not reach sub- or para-aortic stations.
Considering the lymph node staging after EBUS-TBNA, neoadjuvant treatment was indicated in 41 patients (51.3%). The majority of patients (31, 10.1%) underwent cisplatinum based chemotherapy.
The mutational assessment was performed in six patients. Four patients resulted wild type genotyping for epidermal growth factor receptor gene (EGFR) or anaplastic lymphoma kinase gene (ALK) and underwent chemotherapy standard treatment. One patient presented v-Raf murine sarcoma viral oncogene homolog B gene (BRAF 1) mutation. One patient had programmed cell death ligand 1 (PD-L1) expression above 55% and received immunotherapy with pembrolizumab antibody with a complete mediastinal lymph node downstaging after therapy.
Mediastinal staging and neoadjuvant treatment data are summarized in Table 2.
Table 2
| Clinical information | N of patients (% of 80) |
|---|---|
| Non-invasive staging | |
| CT scan N2 positivity | 21 (26.3) |
| PET N2 positivity | 11 (13.8) |
| CT scan and PET N2 positivity | 48 (60.0) |
| Goal of the procedure (% of the staged patients) | |
| Diagnosis and staging | 49 (61.3) |
| Mediastinal staging | 14 (17.5) |
| Endobronchial sampling and staging | 5 (6.3) |
| cN3 confirmation | 6 (7.5) |
| Diagnosis, staging and mutational status | 6 (7.5) |
| Sampling | |
| Number of total station | 109 |
| Number of lymph nodes | 136 |
| Station sampled (% of the stations sampled) | |
| #7 | 48.6 |
| #4R | 24.8 |
| #7 together with #4R | 21.1 |
| Others | 5.5 |
| Histology | |
| Adenocarcinoma | 43 (53.8) |
| Squamous cell carcinoma | 29 (36.3) |
| Adenosquamous carcinoma | 1 (1.3) |
| NSCLC | 6 (7.5) |
| Stage after EBUS-TBNA | |
| IIA | 1 (1.3) |
| IIB | 31 (38.8) |
| IIIA | 33 (41.3) |
| IIIB | 14 (17.5) |
| IIIC | 1 (1.3) |
| Neoadjuvant treatment | |
| No | 39 (48.4) |
| Yes | 41 (51.3) |
| Type of treatment | |
| Chemotherapy | 40 (50.0) |
| Chemo-radiotherapy | 1 (1.3) |
| Type of chemotherapy | |
| Cisplatinum based | 31 (10.1) |
| Carboplatin based | 5 (1.6) |
CT, computed tomography; PET, positron emission tomography; NSCLC, non-small-cell lung cancer; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration.
Patients that underwent neoadjuvant treatment showed at final pathological evaluation: 1 patient (1.3%) yIA2 stage, 1 (1.3%) yIB, 9 (11.3%) yIIB, 14 (17.5%) yIIIA, and 13 (16.3%) yIIIB. One patient had a contralateral progression of the disease (final stage pN3–yIIIC) which was not detectable by the imaging restaging before surgery. A lymph node downstaging promoted by the neoadjuvant treatment was observed in 11 cases of the 41 neoadjuvant treated patients (26.8%). These data are summarized in Table 3.
Table 3
| Surgical procedure information | N of patients (% of 80) |
|---|---|
| Side of procedure | |
| Right | 45 (56.3) |
| Left | 35 (43.8) |
| Type of surgery | |
| Standard pneumonectomy | 68 (85.0) |
| Extended pneumonectomy | 46 (57.5) |
| Sleeve pneumonectomy | 3 (3.8) |
| Extended sleeve pneumonectomy | 10 (12.5) |
| Pathological stage | |
| IIB | 7 (8.8) |
| IIIA | 28 (35.0) |
| IIIB | 4 (5.0) |
| yIA2 | 1 (1.3) |
| yIB | 1 (1.3) |
| yIIB | 9 (11.3) |
| yIIIA | 14 (17.5) |
| yIIIB | 13 (16.3) |
| yIIIC | 1 (1.3) |
Surgical resection included 68 standard pneumonectomies (85%), 46 extended resections (57.5%), for pericardium or superior vena cava involvement, 3 pneumonectomies with bronchoplastic (3.8%) and 10 extended pneumonectomies for pericardium, superior vena cava, atrium or diaphragm resection with bronchoplastic (12.5%).
Recurrence were detected at follow-up in 20 patients (25%), 7 cases of local recurrence and 13 distant metastases (with brain and bone the most frequent sites of recurrence). After a median follow-up of 2-year, 63 patients (78.8%) were alive while 17 patients (21.3%) were dead due to progression of the disease (Table 4).
Table 4
| Follow-up data | N of patients (% of 80) |
|---|---|
| Recurrence of the disease | |
| No | 60 (75.0) |
| Yes | 20 (25.0) |
| Site of recurrence | |
| Regional | 7 (35.0) |
| Distant | 13 (65.0) |
| Follow-up information | |
| Not evidence of the disease | 54 (67.5) |
| Alive with the disease | 9 (11.3) |
| Dead from the disease | 17 (21.3) |
| Survival, months, average [range] | 21.9 [0–85] |
Discussion
Lung cancer remains the leading cause of cancer-related deaths, mostly due to asymptomatic onset leading to diagnosis delay. In the majority of cases, lung cancer is a locally advanced tumor at the time of diagnosis, often metastasized to the lymph nodes or invading the adjacent hilar and mediastinal structures.
The cohort analyzed in this study had advanced disease requiring a major surgical procedure, like pneumonectomy. Mediastinal staging is pivotal to an adequate treatment and clinical staging alone is often not enough to stratify patients’ candidate for surgery and invasive mediastinal staging is often required. Mediastinoscopy has been considered the gold standard for mediastinal staging for many years but is was ever been underused (11) due to its invasively and risk of complications (9,12).
In the last years, a minimally invasive procedure was developed and, in a short period of time, EBUS-TBNA revolutionized the approach to lung cancer diagnosis and staging and became the first approach in many oncological settings.
For many years, mediastinoscopy was the procedure of choice at our Division for invasive mediastinal staging.
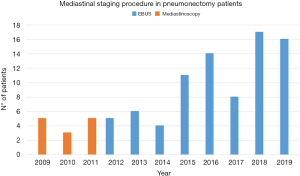
Since 2011, we started performing EBUS-TBNA for the diagnosis and mediastinal staging of lung cancer patients and other clinical indications (Figure 1). The less invasively and the high diagnostic accuracy of the procedure make it our first choice in many different clinical situations (7).
In this study, we evaluated the role of EBUS-TBNA in a selected group of patients candidates to a very invasive surgical procedure as a pneumonectomy for lung cancer treatment.
Between the 80 patients included in the study, all patients underwent clinical radiological staging with CT and PET scans. Surprisingly, 51 (63.8%) presented a different stage after EBUS-TBNA when compared to the clinical stage after CT and PET scans.
In 51 patients’ image methods upper staged the disease (cIIIA and cIIIB stages) and the results of EBUS-TBNA excluded the suspected lymph node involvement. The results of EBUS-TBNA allowed an aggressive surgical approach with curative intent in the majority of patients.
Between the 80 patients, 41 patients underwent neoadjuvant chemotherapy according to EBUS-TBNA cell typing and molecular analysis results.
In one patient diagnosed with an advanced stage squamous cell carcinoma with carina invasion and contralateral lymph node involvement (IIIC), a high expression level of the PD-L1 was highlighted by EBUS-TBNA lymph node samples and immunotherapy with pembrolizumab antibody was performed with a complete lymph node down stage after surgery (ypT1 ypN0).
In five patients EBUS-TBNA was used also performed to evaluate bronchial involvement and the extension of the disease.
In fact, imaging methods such as CT scan, are often unable to predict the extent of tumor invasion of an adjacent area such as the main bronchus. In a comparative study between EBUS and CT scan, the first was shown to be superior (accuracy of 94% compared to 51% respectively) in identifying airway involvement by lung cancers (13).
In 49 patients EBUS-TBNA was performed for cell typing and staging, in 6 patients for cell typing, mediastinal staging and molecular analysis confirming its strategic role as an “all in one” procedure with a very high diagnostic accuracy (Figure 2).

In the present study EBUS-TBNA sensibility, negative predictive value and diagnostic accuracy were 86.7%, 94.4% and 95.9% respectively. These data are in accordance with our previous findings and the literature available data (7,8).
In the present study, EBUS-TBNA changed completely the treatment option in 61.8% of the patients confirm its strategic role in this group of patients, allowing aggressive treatment with curative intent and the best possible survival rates.
The present study has limitations due to its retrospective nature. First, due to its retrospective nature, not all patients underwent the same type of staging protocol and consecutive pre-operative treatment. The presence of advanced cases in the first years of the analyzed data can be reflect the low accuracy of methods used. Moreover, EBUS-TBNA procedures have increased in number and aim progressively in the last years.
In summary, in patients with lung cancer that require major surgical procedures, such as pneumonectomy, mediastinal staging plays an essential role, which must be accurate and less invasive. Among the different approaches, EBUS-TBNA has a leading role and since its introduction in clinical routine, it has evolved from a merely diagnostic tool to a simple rapid procedure able to globally characterize the disease without altering the surgical field and free of major complications (Figure 2).
In patients’ candidate to pneumonectomy, EBUS-TBNA demonstrated a strategic role changing completely the treatment approach in this group of patients.
Acknowledgments
This article was revised by Susan Jane West (Professional translator/editor).
Funding: This work was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5×1,000 funds.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Lorenzo Spaggiari, Luca Bertolaccini) for the series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2020.03.07). The series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” was commissioned by the editorial office without any funding or sponsorship. FP serves as an unpaid editorial board member of Shanghai Chest from Jun 2018 to May 2020. LS serves as an unpaid editorial board member of Shanghai Chest from Aug 2019 to Jul 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee approved data collection and analysis and waived the need for written consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
-
World Health Organization - Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet 2000;355:479-85. [Crossref] [PubMed]
- Spaggiari L, Casiraghi M, Guarize J, et al. Outcome of patients with pN2 "potentially resectable" nonsmall cell lung cancer who underwent surgery after induction chemotherapy. Semin Thorac Cardiovasc Surg 2016;28:593-602. [Crossref] [PubMed]
- Skrzypczak PJ, Roszak M, Kasprzyk M, et al. Pneumonectomy - permanent injury or still effective method of treatment? Early and long-term results and quality of life after pneumonectomy due to non-small cell lung cancer. Kardiochir Torakochirurgia Pol 2019;16:7-12. [Crossref] [PubMed]
- Sakairi Y, Nakajima T, Yoshino I. Role of endobronchial ultrasound-guided transbronchial needle aspiration in lung cancer management. Expert Rev Respir Med 2019;13:863-70. [Crossref] [PubMed]
- Liam CK, Andarini S, Lee P, et al. Lung cancer staging now and in the future. Respirology 2015;20:526-34. [Crossref] [PubMed]
- Guarize J, Casiraghi M, Donghi S, et al. Endobronchial ultrasound transbronchial needle aspiration in thoracic diseases: much more than mediastinal staging. Can Respir J 2018;2018:4269798 [Crossref] [PubMed]
- Dong X, Qiu X, Liu Q, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the mediastinal staging of non-small cell lung cancer: a meta-analysis. Ann Thorac Surg 2013;96:1502-7. [Crossref] [PubMed]
- Asano F, Aoe M, Ohsaki Y, et al. Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration: a nationwide survey by the Japan Society for Respiratory Endoscopy. Respir Res 2013;14:50. [Crossref] [PubMed]
- Guarize J, Pardolesi A, Donghi S, et al. Endobronchial ultrasound for mediastinal staging in lung cancer patients. Multimed Man Cardio-Thoracic Surg 2014;2014:1813-975. [PubMed]
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6; discussion 2056. [Crossref] [PubMed]
- Dietrich CF, Annema JT, Clementsen P, et al. Ultrasound techniques in the evaluation of the mediastinum, part I: endoscopic ultrasound (EUS), endobronchial ultrasound (EBUS) and transcutaneous mediastinal ultrasound (TMUS), introduction into ultrasound techniques. J Thorac Dis 2015;7:E311-25. [PubMed]
- Herth F, Ernst A, Schulz M, et al. Endobronchial ultrasound reliably differentiates between airway infiltration and compression by tumor. Chest 2003;123:458-62. [Crossref] [PubMed]
Cite this article as: Guarize J, Sedda G, Bonizzoni G, Donghi SM, Casiraghi M, Petrella F, Spaggiari L. The role of endobronchial ultrasound transbronchial needle aspiration in patients candidate to pneumonectomy. Shanghai Chest 2020;4:39.


