Lung cancer surgery in octogenarians: a meta-analysis of predictors for postoperative complications
Highlight box
Key findings
• A good preoperative PS, VATS approach and sublobar resections leads to less postoperative complications in octogenarian patients undergoing lung cancer surgery
What is known and what is new?
• Lung cancer surgery is a feasible option in octogenarian patients, but postoperative complication rate remains high.
• This meta-analysis showed the impact of different risk factors for postoperative complications.
What is the implication, and what should change now?
• An accurate selection of octogenarians with a good preoperative PS is mandatory to avoid an high postoperative complication rate. Similarly, VATS approach and sublobar resection should be preferred in those patients if technically and oncologically feasible.
Introduction
Background
The world population continues to grow old and the definition of elderly patients has changed during the last decades from over 65 years to over 70 years and more (1). Due to the health care improvement, lung cancer has progressively become a disease of older people. Nowadays, the median age at diagnosis is over 70 years old with almost 40% of new cases diagnosed in patient aged 75 or more (2). The peak of incidence in the U.S. is calculated to range between 80 and 84 years old with an age-specific incidence rate of about 400/100,000. Therefore, the amount of octogenarians with early-stage non-small cell lung cancer (NSCLC), eligible for surgery, has increased and about 14% of all resectable NSCLC concern patient aged 80 or above.
While in 19th century age over 80 represented a contraindication to pulmonary resection per se (3), in the last decades a high number of reports and trials have focused on the feasibility of lung cancer surgery in octogenarians (4-8). In fact, the expectation of life at the age of 80 is about 9.3 years (9,10) suggesting that the lifetime limiting factor for octogenarians with resectable lung cancer is not the age but the cancer itself.
Rationale and knowledge gap
Recent studies concerning lung cancer surgery in octogenarians have argued previous reports of prohibitively high morbidity and mortality rate in such age conditions (11), showing good results about short and long-term survival. Actually, the 3-year survival rate range between 51% and 83% in selected patients, suggesting that surgical resection is safe and feasible even in patient aged 80 or more (Table 1).
Table 1
| Author | Year of publication | Number of patients | Complication rate (%) |
30 day mortality (%) | Survival rate (%), 1 y – 3 y – 5 y |
|---|---|---|---|---|---|
| Aoki et al. (12) | 2000 | 35 | 60 | 0 | 38.9 (5 y) |
| Berry et al. (13) | 2011 | 193 | 46 | 3.6 | NR |
| Brock et al. (14) | 2004 | 68 | 44 | 8,8 | 73 – 51 – 34 |
| Brokx et al. (15) | 2007 | 124 | NR | 4 | 83 – 69 – 47 |
| Dell’Amore et al. (16) | 2015 | 73 | 41 | 2.7 | 96 – 83 – 60 |
| Dillman et al. (8) | 2009 | 70 | NR | 1.4 | 63 (5 y) |
| Dominguez-Ventura et al. (17) | 2006 | 379 | 48 | 6.3 | NR |
| Fanucchi et al. (18) | 2011 | 82 | 30 | 2.4 | 90 – 44 – 36 |
| Guerra et al. (19) | 2013 | 57 | 33.3 | 1.8 | NR |
| Hanagiri et al. (20) | 1999 | 18 | 50 | 0 | 42 (5 y) |
| Hope et al. (5) | 2007 | 20 | 45 | 10 | 59 – 39 – NR |
| Hino et al. (7) | 2015 | 94 | 27.7 | 1.1 | 57.5 (5 y) |
| Igai et al. (21) | 2009 | 95 | 21 | 0 | NR |
| Zhang et al. (22) | 2012 | 52 | 44.2 | 3.8 | 87.1 – 59.8 – 19.1 |
| Mun et al. (6)$ | 2008 | 55 | 25.6 | 3.6 | 65.9 (5 y) |
| Naunheim et al. (11) | 1994 | 37 | 45 | 16 | 30 (5 y) |
| Okami et al. (23) | 2009 | 367 | 8.4 | 1.4 | 56 (5 y) |
| Pagni et al. (24) | 1997 | 54 | 42 | 3.7 | 86 – 62 – 43 |
| Port et al. (25) | 2004 | 61 | 38 | 1.6 | 38 (5 y) |
| Port et al. (3) | 2011 | 121 | 35/63^ | 0/2.5^ | 56.6 (5 y) |
| Tanita et al. (26) | 1999 | 24 | 45.8 | 0 | NR |
| Voltolini et al. (27) | 2009 | 96 | 44 | 9.4 | NR – 51 – 34 |
$, only I stage lung cancer; ^, VATS vs. open. y, year; NR, not reported; VATS, video-assisted thoracoscopic surgery.
However, the postoperative complication rate for those patients remains quietly high with an average of 40% in the most recent studies (Table 1). Some works had analyzed the role of various preoperative predictors of morbidity in octogenarian patients underwent pulmonary resection for lung cancer (12-14,16-18,27-30). However, the lack of meta-analysis does not allow to easily compare the outcomes of these studies that often report discordant results.
Objective
This meta-analysis targets matching the role of preoperative factors in lung cancer surgery for octogenarian patients and tries to attribute to each factor its role as predictor of postoperative complications. We present the following article in accordance with the PRISMA reporting checklist (available at https://shc.amegroups.com/article/view/10.21037/shc-22-45/rc).
Methods
Data source and search strategy
Literature research was performed independently by 2 authors (MB and EM) through PubMed and MEDLINE to identify trials concerning predictors of postoperative complications in octogenarians undergoing lung cancer resection for pulmonary malignancy. The terms “lung cancer”, “non-small cell lung cancer” or “lung cancer surgery” were entered as keywords with “octogenarian” or “80 years” both independently and in multiple combinations. Manual research on articles regarding lung cancer surgery in elderly patients was also performed. Clinical studies involving patients aged 80 or more undergoing lung cancer surgery between 1990 and 2020 were screened. Only clinical studies explicitly appraising risk factors for postoperative complications, and providing adjusted risk effect estimates, were included. Only articles published in English language were considered.
Selection criteria
Published studies were considered eligible if they explicitly reported a correlation between postoperative complications and preoperative factors in terms of P value either in univariate or multivariate analysis. Two authors (MB and EM) independently screened the studies. When the results of a study were reported in more than one publication, the most informative publication was considered. Case reports, case series, reviews, editorials and commentaries were excluded. When there was no consensus, disagreements were resolved through referral to a third reviewer (MA).
Data extraction and risk of bias assessment
The data were extracted by two independent reviewers (MB and EM) including study design, publication year, number of participants, age, preoperative characteristics, type of surgical intervention, type of postoperative complications, postoperative complication rate and statistical outcomes. Risk of bias was assessed using the Quality in Prognostic Factor Studies (QUIPS) tool for each study (https://cdn.amegroups.cn/static/public/shc-22-45-1.pdf). Two different authors (RVZ and FF) independently evaluated the risk for each study completing each domain of QUIPS tool. Differences were resolved through referral to a third author (MB), if any.
Statistical analysis
P values from individual studies were analyzed to assess the role of each factor as a predictor of postoperative complications. For the combination of P values, the Fisher method (31) was used, as implemented by the fisherp function from the corto R package (32). A fixed value of P=1 was used for works where P values were reported as “P>0.05”. A pooled two-tailored P value ≤0.05 was considered statistically significant. Statistical analysis was performed using r-project platform (R version 3.5.1) and graphical elaboration by Inkscape (Inkscape project, version 1.0.2) (33).
Results
Study selection and characteristics
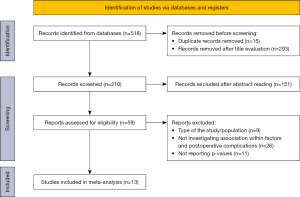
Our search generated 518 records for evaluation. After exclusion of duplicates and irrelevant articles by title, 210 articles remained for further evaluation. Those records were screened for inclusion by abstract reading, resulting in 59 potentially relevant papers. After full-text evaluation, a final set of 13 cohort studies, including 2,596 patients, explicitly appraising risk factors for postoperative complications and providing adjusted risk effect estimates was included in the dataset for inferential analysis (11-14,16-18,23,25,27-30). Among these articles, eight reported operative and postoperative data from a single institution (12-14,17,18,25,27,28), three from multicentric database (11,16,30) and two from national registry (23,29). All studies were retrospective, except for one nationwide multicenter prospective cohort (30). The search process is illustrated in Figure 1.
Risk of bias
All the studies were classified on the basis of subjective assessment using the QUIPS tool. Each domain was evaluated as high, moderate, and low risk of bias and an overall risk has been calculated (https://cdn.amegroups.cn/static/public/shc-22-45-1.pdf, Table 2). All studies have been considered at low risk.
Table 2
| Name | PS | FEV1 | Resection | Approach | Tobacco | Male |
|---|---|---|---|---|---|---|
| Aoki et al. (12) | >0.05d | >0.05 | >0.05j | |||
| Berry et al. (13) | 0.006 | 0.001 | 0.0001 | 0.96 | ||
| Brock et al. (14) | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | |
| Dell’Amore et al. (15) | 0.01a | 0.001 | ||||
| Dominguez- Ventura et al. (16) | 0.47h | 0.02i | 0.01k | 0.03 | ||
| Fanucchi (17) | 0.0033c | 0.458f | 0.187 | 0.0832 | 0.0236 | |
| Ito et al. (18) | 0.85 | 0.25 | 0.13 | 0.06 | ||
| Naunheim et al. (11) | >0.05e | >0.05 | >0.05 | |||
| Okami et al. (19) | 0.0001b | 0.857 | 0.528 | |||
| Port et al. (20) | 0.252a | 0.591 | ||||
| Saha et al. (21) | 0.0001e | 0.0001 | ||||
| Saji et al. (22) | 0.59 | 0.065 | 0.001 | 0.001 | ||
| Voltolini et al. (23) | 0.042b | 0.36g | 0.008l | 0.18 | ||
| Fisher analysis | 0.004 | 0.109 | 0.002 | 0.007 | 0.399 | 0.053 |
a, comorbidity >1; b, comorbidity Y/N; c, ACE-27; d, FEV1/BSA; e, COPD diagnosis; f, FEV1<1.5 L; g, FEV1 <60%; h, FEV1 between 40%<x<70%; i, only lobe/bilobectomy; j, Brinkman index; k, between 20–80 pack/year; l, only bilobectomy and pneumonectomy. PS, performance status > II; FEV1, forced expiratory volume in 1 second; Resection, pulmonary resection greater than wedge; Approach, thoracotomy vs. VATS; BSA, body surface area; VATS, video-assisted thoracoscopic surgery.
Results of individual studies
More than 20 potential predictors were screened and adjusted P values for postoperative complications were entered in the dataset. Either P values resulting from univariate analysis and multivariate Cox regression analysis were considered. Finally, only predictors reported at least in 30% of the studies were included, limiting the inferential analysis to the six most frequently reported parameters: performance status (PS) or similar, forced expiratory volume in 1 second (FEV1), type of resection, surgical approach, history of tobacco abuse and male gender.
Detailed effect estimates were reported for PS or similar by 5 studies (38%), for FEV1 by 7 (54%), for type of resection by 8 (62%), for surgical approach by 4 (31%) for history of tobacco abuse by 4 (31%) and for male gender by 6 (46%). Furthermore, non-significant outcomes, reported as “P value >0.05”, were reported for PS in 1 (8%) study, for FEV1 in 3 (23%), for type of resection in 3 (23%), for history of tobacco abuse in 2 (15%) and for male gender in 2 (15%). Finally, type of resection was the most reported predictors (11 studies, 85%), followed by FEV1 (10 studies, 77%), male gender (8 studies, 62%), PS and history of tobacco abuse (6 studies, 46%) and surgical approach (4 studies, 31%) (Table 2). In our dataset, PS is statistically associated with postoperative complications in 4/6 studies, FEV1 in 2/10 studies, type of resection in 4/10, thoracotomy approach, compared to video-assisted thoracoscopic surgery (VATS) approach, in 2/4 studies, history of tobacco abuse in 2/6 studies and male gender in 3/7 studies.
Results of meta-analysis
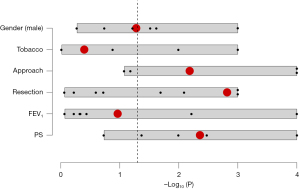
P values from individual studies was combined using Fisher method. Results are showed in Table 2 and Figure 2. A significant association with postoperative complications in octogenarian patients was found for PS (cumulative P value 0.004), type of resection (cumulative P value 0.002), and surgical approach (cumulative P value 0.007). No statistically significant association after Fisher method was found between postoperative complication rate and preoperative FEV1 (cumulative P value 0.109), history of tobacco abuse (cumulative P value 0.399) and male gender (cumulative P value 0.053).
Discussion
Considering the aging of the population and the increasingly frequent diagnosis of lung cancer in advanced age, lots of reports and trials have focused on the role of lung cancer surgery in octogenarians. Recent studies demonstrated that age cannot be considered per se a contraindication for lung cancer surgery, showing good results in short and long-term survival (4-8,34,35).
Preoperative evaluation of these patients is imperative in order to correctly select those patients who will possibly take advantage from surgical treatment. In fact, the high incidence of postoperative complications represents a limit for old patients that often are referred to other treatment even if the neoplasm can be completely eradicated (36).
Key findings
The aim of the present study is to assess the role of each predictor of postoperative complications in octogenarians undergoing lung cancer surgery to help selecting those patients which can get benefit from surgical treatment.
Several factors have been proposed as predictors of postoperative complications in elderly patients. In our study, the inferential analysis has been limited to the six most frequent reported factors: PS, FEV1, type of resection, surgical approach, history of tobacco abuse and male gender.
A PS > II, as reported by American Society of Anesthesiology (ASA) PS score, has been considered as cut off value for postoperative complications in our analysis. However, the majority of studies does not report ASA score in their analysis but analogous classifications. In particular, Dell’Amore et al. (16) and Port et al. (25) analyzed the role of comorbidity >1 as predictor of postoperative complication while Voltolini et al. (27) and Okami et al. (23) considered comorbidity yes/no as cut off in their analysis. Finally, Fanucchi et al. (18) utilized the ACE-27 comorbidity index. Even if the ASA score was not reported, all of these studies focused on the importance of preoperative PS as predictor of postoperative complications in octogenarians. As all those classifications can be assumed to be analogous to ASA score > II, they have been included in the analysis.
Preoperative FEV1 was one of the most reported predictors. Its association with postoperative complications was assessed with continuous values in 4 studies (13,14,25,28) in terms of chronic obstructive pulmonary disease (COPD)/obstructive airway disease in 2 (11,29), with FEV1 <1.5 L in 1 (18), with FEV1 between 40% and 70% (17), FEV1 <60% in 1 (27) in 1 and as FEV1/body surface area (BSA) in 1 (12). In one study, only the association with respiratory complication was assessed (12).
Concerning type of resection, most studies explored the relationship between postoperative complications and pulmonary resection greater than wedge (11-14,16-18,23,28,27,30). However, Dominguez-Ventura et al. (17) did not considered pneumonectomy in his analysis while Voltolini et al. (27) analyzed the relationship between postoperative complication and pulmonary resection greater than lobectomy.
Other predictors such as cancer histology, operative time, body mass index (BMI), diffusing capacity of the lungs for carbon monoxide (DLCO) and others were not uniformly reported in literature and thus were not included in this meta-analysis according to our selection criteria (e.g., material and methods section).
Implications and actions needed
Our analysis confirms that an accurate preoperative and operative evaluation of octogenarians undergoing lung cancer surgery is essential to avoid a prohibitive postoperative complication rate. In particular, preoperative PS should be evaluated in the therapeutic decision process more than age per se considering that is associated with a significantly lower complication rate. Similarly, VATS approach should be the preferred surgical technique in octogenarian patients since its lower impact and faster postoperative course. Moreover, concerning the type of pulmonary resection, sublobar resection should be preferred if technically and oncologically feasible. In fact octogenarians remain frail patients regardless the preoperative PS and comorbidities; thus, a less invasive surgery, if oncologically effective, could be convenient resulting in a faster and smoother postoperative course. Moreover, recent trials showed that sub-lobar resections are oncologically non-inferior to lobectomy in selected patients (37). Interestingly, impaired lung function was not significantly associated to post-operative complications in our analysis. This is probably due to the patient selection for surgery. In fact, octogenarians with an impaired lung function are likely to be referred to treatment options other than surgery.
Strengths and limitations
This meta-analysis presents some limitations mainly due to the heterogeneously of the selected studies in terms of predictors and outcomes, the presence of numerous missing values or unreported non-significant P values and finally the poor number of studies about this topic. Moreover, the impact of other predictors has not been evaluated. Larger studies are needed to confirm our results. However, this study could provide important information for the preoperative management of octogenarians undergoing lung cancer surgery.
Conclusions
Surgery is nowadays a feasible option in octogenarian patients with early-stage lung cancer. An accurate patient selection is mandatory considering that postoperative complication rate for those patients remains relatively high. According to this comprehensive review and meta-analysis, the most important predictors for postoperative complications were the extension of pulmonary resection, surgical approach and preoperative PS.
Acknowledgments
Funding: FMG is funded by the Italian Ministry of University and Research, PNRR program, HPC, Big Data and Quantum Computing project.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-22-45/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-22-45/coif). MB serves as an unpaid editorial board member of Shanghai Chest from July 2022 to June 2024. FV serves as an unpaid editorial board member of Shanghai Chest from July 2017 to June 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Orimo H, Ito H, Suzuki T, et al. Reviewing the definition of “elderly”. Geriatr Gerontol Int 2006;6:149-58. [Crossref] [PubMed]
- SEER Cancer Stat Facts: Lung and Bronchus Cancer. National Cancer Institute. Bethesda, MD. Available online: https://seer.cancer.gov/csr/1975_2015/sections.html
- Weiss W. Operative mortality and five-year survival rates in men with bronchogenic carcinoma. Chest 1974;66:483-7. [Crossref] [PubMed]
- Port JL, Mirza FM, Lee PC, et al. Lobectomy in octogenarians with non-small cell lung cancer: ramifications of increasing life expectancy and the benefits of minimally invasive surgery. Ann Thorac Surg 2011;92:1951-7. [Crossref] [PubMed]
- Hope WW, Bolton WD, Kalbaugh CA, et al. Lung cancer resection in octogenarians: a reasonable approach for our aging population. Am Surg 2007;73:22-4. [Crossref] [PubMed]
- Mun M, Kohno T. Video-assisted thoracic surgery for clinical stage I lung cancer in octogenarians. Ann Thorac Surg 2008;85:406-11. [Crossref] [PubMed]
- Hino H, Murakawa T, Ichinose J, et al. Results of Lung Cancer Surgery for Octogenarians. Ann Thorac Cardiovasc Surg 2015;21:209-16. [Crossref] [PubMed]
- Dillman RO, Zusman DR, McClure SE. Surgical resection and long-term survival for octogenarians who undergo surgery for non-small-cell lung cancer. Clin Lung Cancer 2009;10:130-4. [Crossref] [PubMed]
- Dati ISTAT, Italian Statistical National institute, Life Table (Accessed on 11th Aug 2022); Available online: http://dati.istat.it/Index.aspx?DataSetCode=DCIS_MORTALITA1&Lang=en
- Arias E, Heron M, Xu J. United States Life Tables, 2013. Natl Vital Stat Rep 2017;66:1-64. [PubMed]
- Naunheim KS, Kesler KA, D'Orazio SA, et al. Lung cancer surgery in the octogenarian. Eur J Cardiothorac Surg 1994;8:453-6. [Crossref] [PubMed]
- Aoki T, Yamato Y, Tsuchida M, et al. Pulmonary complications after surgical treatment of lung cancer in octogenarians. Eur J Cardiothorac Surg 2000;18:662-5. [Crossref] [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. A model for morbidity after lung resection in octogenarians. Eur J Cardiothorac Surg 2011;39:989-94. [Crossref] [PubMed]
- Brock MV, Kim MP, Hooker CM, et al. Pulmonary resection in octogenarians with stage I nonsmall cell lung cancer: a 22-year experience. Ann Thorac Surg 2004;77:271-7. [Crossref] [PubMed]
- Brokx HA, Visser O, Postmus PE, et al. Surgical treatment for octogenarians with lung cancer: results from a population-based series of 124 patients. J Thorac Oncol 2007;2:1013-7. [Crossref] [PubMed]
- Dell'Amore A, Monteverde M, Martucci N, et al. Lobar and sub-lobar lung resection in octogenarians with early stage non-small cell lung cancer: factors affecting surgical outcomes and long-term results. Gen Thorac Cardiovasc Surg 2015;63:222-30. [Crossref] [PubMed]
- Dominguez-Ventura A, Allen MS, Cassivi SD, et al. Lung cancer in octogenarians: factors affecting morbidity and mortality after pulmonary resection. Ann Thorac Surg 2006;82:1175-9. [Crossref] [PubMed]
- Fanucchi O, Ambrogi MC, Dini P, et al. Surgical treatment of non-small cell lung cancer in octogenarians. Interact Cardiovasc Thorac Surg 2011;12:749-53. [Crossref] [PubMed]
- Guerra M, Neves P, Miranda J. Surgical treatment of non-small-cell lung cancer in octogenarians. Interact Cardiovasc Thorac Surg 2013;16:673-80. [Crossref] [PubMed]
- Hanagiri T, Muranaka H, Hashimoto M, et al. Results of surgical treatment of lung cancer in octogenarians. Lung Cancer 1999;23:129-33. [Crossref] [PubMed]
- Igai H, Takahashi M, Ohata K, et al. Surgical treatment for non-small cell lung cancer in octogenarians--the usefulness of video-assisted thoracic surgery. Interact Cardiovasc Thorac Surg 2009;9:274-7. [Crossref] [PubMed]
- Zhang J, Xue ZQ, Chu XY, et al. Surgical treatment and prognosis of octogenarians with non-small cell lung cancer. Asian Pac J Trop Med 2012;5:465-8. [Crossref] [PubMed]
- Okami J, Higashiyama M, Asamura H, et al. Pulmonary resection in patients aged 80 years or over with clinical stage I non-small cell lung cancer: prognostic factors for overall survival and risk factors for postoperative complications. J Thorac Oncol 2009;4:1247-53. [Crossref] [PubMed]
- Pagni S, Federico JA, Ponn RB. Pulmonary resection for lung cancer in octogenarians. Ann Thorac Surg 1997;63:785-9. [Crossref] [PubMed]
- Port JL, Kent M, Korst RJ, et al. Surgical resection for lung cancer in the octogenarian. Chest 2004;126:733-8. [Crossref] [PubMed]
- Tanita T, Hoshikawa Y, Tabata T, et al. Functional evaluations for pulmonary resection for lung cancer in octogenarians. Investigation from postoperative complications. Jpn J Thorac Cardiovasc Surg 1999;47:253-61. [Crossref] [PubMed]
- Voltolini L, Rapicetta C, Ligabue T, et al. Short- and long-term results of lung resection for cancer in octogenarians. Asian Cardiovasc Thorac Ann 2009;17:147-52. [Crossref] [PubMed]
- Ito H, Nakayama H, Yamada K, et al. Outcomes of lobectomy in 'active' octogenarians with clinical stage I non-small-cell lung cancer. Ann Thorac Cardiovasc Surg 2015;21:24-30. [Crossref] [PubMed]
- Saha SP, Bender M, Ferraris VA, et al. Surgical treatment of lung cancer in octogenarians. South Med J 2013;106:356-61. [Crossref] [PubMed]
- Saji H, Ueno T, Nakamura H, et al. A proposal for a comprehensive risk scoring system for predicting postoperative complications in octogenarian patients with medically operable lung cancer: JACS1303. Eur J Cardiothorac Surg 2018;53:835-41. [Crossref] [PubMed]
- Elston RC. On Fisher's Method of Combining p-Values. Biom J 1991;33:339-45. [Crossref]
- Mercatelli D, Lopez-Garcia G, Giorgi FM. corto: a lightweight R package for gene network inference and master regulator analysis. Bioinformatics 2020;36:3916-7. [Crossref] [PubMed]
- Giorgi FM, Ceraolo C, Mercatelli D. The R Language: An Engine for Bioinformatics and Data Science. Life (Basel) 2022;12:648. [Crossref] [PubMed]
- Vaz Souza R, Bassi M, Mantovani S, et al. Comparison of preoperative scores predicting outcome in elderly undergoing lung malignancies resection. J Thorac Dis 2020;12:7083-8. [Crossref] [PubMed]
- Ganti AK, Shostrom V, Alorabi M, et al. Early Stage Non-Small-Cell Lung Cancer in Octogenarian and Older Patients: A SEER Database Analysis. Clin Lung Cancer 2016;17:285-91. [Crossref] [PubMed]
- Anile M, Poggi C, Diso D, et al. Do the right thing! J Thorac Dis 2019;11:S266-7. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
Cite this article as: Bassi M, Vannucci J, Giorgi FM, Vaz Souza R, Ferrante F, Bianco M, Mottola E, De Giacomo T, Diso D, Poggi C, Venuta F, Anile M. Lung cancer surgery in octogenarians: a meta-analysis of predictors for postoperative complications. Shanghai Chest 2023;7:14.