
Lung cancer infiltrating the chest wall: a narrative review
Introduction
Lung cancer is leading cause of cancer-related death worldwide. Despite that in recent years, the implementation of screening programs has facilitated the detection of early-stage lung cancer, over 5% of non-small cell lung cancer (NSCLC) cases are diagnosed at a locally advanced stage (1).
The 8th edition of the tumor-node-metastasis (TNM) staging system by the American Joint Committee on Cancer (AJCC) defined lung cancer infiltrating the chest wall (CW) as T3 stage (or T4 if the infiltration involved the vertebral body); this classification includes several scenarios, which require multidisciplinary evaluation for treatment (2).
The cornerstone of treatment for lung cancer with CW infiltration has always been surgical en bloc resection. The first successful en bloc CW resection was published by Coleman in 1947 (3); in subsequent decades, several advancements in technical approach for resection and reconstruction of the CW were proposed. However, despite that over the years, combinations of chemotherapy and radiotherapy treatment have been proposed, as adjuvant and neoadjuvant treatments, the most important prognostic factors remain R0 surgical resection, and lymph node stage.
This narrative review aims to systematically describe the diagnostic pathway, the therapeutic indications, and the main surgical techniques in patients with lung cancer with CW infiltration. We present this article in accordance with the Narrative Review reporting checklist (available at https://shc.amegroups.com/article/view/10.21037/shc-23-24/rc).
Methods
We conducted a search of the PubMed database for articles published between January 2018 and December 2022, using the following keywords: “lung cancer infiltrating chest wall”, “chest wall resection”, “chest wall reconstruction”, and “rib resection”. The search was supplemented by a manual search of published reviews according to a strategy designed and constructed by the authors (Table 1). Acceptable literature included original articles, case reports, case series, and retrospective studies. Articles published in languages other than English were excluded.
Table 1
| Items | Specification |
|---|---|
| Date of search | 21st March 2023 |
| Databases and other sources searched | PubMed |
| Search terms used | “Lung cancer infiltrating chest wall”, “chest wall resection”, “chest wall reconstruction”, and “rib resection” |
| Timeframe | January 2018 to December 2022 |
| Inclusion and exclusion criteria | Inclusion criteria: original articles, case reports, case series, and retrospective studies |
| Exclusion criteria: all non-English language articles | |
| Selection process | Both authors performed article selection independently |
Discussion
Preoperative oncological evaluation
Just as for all patients with primary lung cancer, adequate staging is paramount, particularly when the CW is invaded by the tumor. Patients should undergo a computed tomography (CT) scan to evaluate tumor localization and local infiltration. The use of respiratory dynamic magnetic resonance imaging (MRI), with the demonstration of the independent CW and lung movements during breathing, may be useful to investigate a possible parietal pleura invasion. 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) can be used to study the lymph node metabolism and clinical stage of disease and can assist clinical decision making.
Given the locally advanced nature of these patients, nodal staging must be as accurate as possible. The incidence of nodal involvement ranged from 15% to 31% in large series of T3 lung cancer cases and the impact of N+ on survival was reported as significant; the 5-year overall survival (OS) of N2 patients ranged from 6% to 17%, whereas was over 40% in N0 disease (4). For these reasons, invasive lymph node staging is mandatory to determine correct treatment. Endobronchial ultrasound (EBUS) and endoscopic ultrasound (EUS) are feasible and have high sensitivity and specificity to detect nodal involvement. In 2015, the European Respiratory Society/European Society of Gastrointestinal Endoscopy/European Society of Thoracic Surgeons published a new guideline for diagnosis and staging of lung cancer: for mediastinal nodal staging, endosonography is recommended over surgical staging as the initial procedure, with recommendation grade A (5). When necessary, mediastinoscopy should be considered to rule out the possibility of lymph-nodal metastasis.
For preoperative histological diagnosis, it is preferable that patients undergo a preoperative biopsy performed by an interventional radiologist. Fine needle biopsy is often enough to distinguish primary lung cancer from secondary lesions or primary CW tumors, but continuing consultation with the pathologist at the time of biopsy and surgery is mandatory to ensure an accurate diagnosis and treatment.
Chemo-radiotherapy treatment
According to the 8th edition of TNM classification, lung cancer that infiltrates the CW is classified as T3; lymph node involvement determines complete staging and consequently the appropriate treatment strategy. The current European Society for Medical Oncology (ESMO) guidelines (6) recommend adjuvant chemotherapy for patients with stage IIB tumors or more (level of evidence I, grade of recommendation A), including patients with CW invasion, with or without nodal metastasis. In 2018, Drake et al. investigated the role of adjuvant chemotherapy in patients with completely resected T3N0 NSCLC invading the CW. They reviewed a large database using the National Cancer Database (NCDB) from the USA, and identified 2,326 patients who underwent complete resection of N0 NSCLC with invasion of the CW. In their propensity-matching study, patients who received chemotherapy after surgery had significantly better survival than patients who did not (7). Another review of the NCDB published in 2017 by Ahmad et al. suggested that adjuvant chemotherapy may confer a survival benefit in patients with N0 lung cancer and CW invasion. They showed that in their case studies, patients treated with adjuvant therapy had a 17-month survival benefit compared to untreated patients [hazard ratio (HR): 0.74; 95% confidence interval (CI): 0.6–0.9] (8).
Excluding lung cancer invading the apical CW or thoracic inlet, studies evaluating multimodality therapy for lung cancers invading other parts of the chest are few. If the supporters of induction therapy argue that it may increase the rate of resectability and influence micrometastasis control, the detractors argue that it has been studied only in a Pancoast tumor setting and there are no specific data for other lung cancers that invade the CW (4,9). In 2014, Kawaguchi et al. collected the data of 51 patients with T3N0/1 lung cancer who received chemoradiotherapy (2 cycles of cisplatin/vinorelbine and 40 Gy) followed by surgical en bloc resection, and they identified complete pathologic response in only 25% of patients (10).
In 2019, a worldwide expert panel in thoracic surgery, plastic surgery, and biomedical engineering released the Chinese expert consensus conference on CW tumor resection and CW reconstruction (11); this consensus has provided clinical guidelines for diagnosis and treatment of CW tumors and presented new perspectives on some points of contention. For NSCLC invading the CW, they recommended wide excision with neoadjuvant and/or adjuvant therapy for patients with stage T3–4N0–1M0.
More recently, in a phase 3 randomized trial published in the New England Journal of Medicine (CheckMate 816), the authors investigated the role of immunochemotherapy in a neoadjuvant regime in patients with IB–IIIA lung cancer. The authors randomly assigned patients to receive either nivolumab plus platinum-based chemotherapy or platinum-based chemotherapy alone, followed by resection. The analysis confirmed that the addition of nivolumab to neoadjuvant therapy did not increase the incidence of adverse events or impede the feasibility of surgery; nivolumab plus chemotherapy resulted in significantly longer event-free survival (31.6 vs. 20.8 months, P=0.005) and higher percentage of patients with a pathological complete response than chemotherapy alone (24.0% vs. 2.2%, P<0.001) (12).
Resection and surgical technique
Despite that for many years, CW invasion was considered a contraindication for upfront resection, these days, NSCLC invading the CW is primarily treated with surgery. It is universally accepted that completeness of resection of the CW and nodal status are the most significant prognostic factors in these kinds of patients.
Considering the variety of clinical and radiological presentations, surgical management must be planned according to the tumor size and location.
Traditionally, a lateral approach by thoracotomy is performed for en bloc resection (13). Whether the CW resection is performed initially or after the anatomic lung resection is not important as long as the entire specimen is removed en bloc. When CW resection is performed, a 2 cm margin in all directions is generally accepted (11); however, the evaluation of resection margins during surgery has limitations because it is based on the combination of macroscopic and microscopic analysis of the specimen. Since frozen section is not possible to perform on samples of resected bone, some authors advocate for a margin of 1 intact rib above and below the tumor and a 3–4 cm lateral margin (14).
In addition, the extent of surgical resection depends also on the depth of invasion. For example, for tumors limited to the parietal pleura, extrapleural resection keeping the CW intact is sufficient to achieve complete resection with long-term survival (15). Rarely, the tumor can infiltrate subcutaneous tissue or skin; in these cases, pedicle myocutaneous flaps may be needed to cover the defect. Collaboration with plastic surgeons and multidisciplinary preoperative planning can facilitate reconstruction after CW resection and preserve the soft tissue chosen for the myocutaneous flap.
In the literature, there are many reports on CW resection by video-assisted thoracoscopic surgery (VATS) (16). Typically, CW resections are performed with a large skin incision, but it is also possible to approach from the inner side of CW, instead of the outside. Ribs can be transected with Gigli saws, endoscopic shears, or high-speed drills (17). The benefit of the VATS approach over thoracotomy has been highly questioned. The decision to perform a VATS approach in resectable lung cancer with CW invasion arises from the perception that opening the rib cage with a smaller incision, thus avoiding rib spreading and the incision of other CW tissue, will have a better outcome, with less postoperative pain, yet the same oncologic results as those of open surgery (18).
Although the first report of a multiport VATS with en bloc resection was made by Widman et al. (19) in 2000, it was not until 2013 that Gonzalez-Rivas reported the first uniportal VATS lobectomy with en block resection of the CW, performed with a single anterior incision for VATS lobectomy and posterior approach for CW resection (20).
Hybrid VATS en bloc resection case series have been published in the last decade. In 2012, Berry et al. reported having performed hybrid thoracoscopy in 12 patients with NSCLC with CW invasion: after standard VATS lobectomy and lymphadenectomy, a limited counter incision was made above the tumor under direct view to accurately identify the ribs to be resected (21).
Jaus et al. proposed an alternative method to estimate CW resection using hypodermic needles positioned under direct visualization; then they performed en bloc resection with a second posterior incision without rib spreading (22). These VATS techniques are notable for their absence of ribs spreading and scapula mobilization (Figure 1).
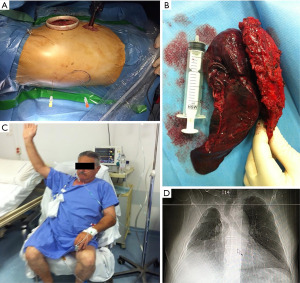
Hennon et al. compared VATS and open chest resection in a retrospective study of 47 patients, 31 of whom had primary NSCLC. Their analysis of postoperative outcomes revealed significantly shorter intensive care unit (ICU) (P=0.028) and hospital stay (P=0.002) in the NSCLC VATS group than in the open surgery group. The stage-matched survival curves for both approaches were superimposable (P=0.88) (23).
Only a few retrospective series have addressed the use of VATS in NSCLC with CW invasion; no trials have compared VATS with the traditional approach, or explored the use of VATS approach in challenging cases, as these cases are mainly treated in high-volume centers.
Reconstruction and surgical technique
CW reconstruction restores the stability of the CW; it is important to maintain protection of underlying organs and avoid flail, scapular tip entrapment, lung herniation, and abnormal lung ventilation.
The decision to reconstruct the CW is based on two parameters: the extent of the defect and its localization. It is generally accepted that CW defects smaller than 5 cm can be managed without reconstruction. An expert consensus in 2019 deemed it necessary to use rigid implants for CW reconstruction for anterolateral CW defects exceeding 5 cm; the CW defects adjacent to the scapula should be reconstructed with rigid implants if the defect exceeds 10 cm to avoid paradoxical breath, disruption of scapulothoracic movements, and scapular tip entrapment (11). In 2015, Puma et al. proposed the division of the chest into critical and non-critical areas: defects localized in the anterior or lateral CW and defects of three or more ribs not covered by the scapula are considered critical and requiring skeletal reconstruction (24). The sternum is always considered a critical area, and it must be reconstructed with rigid material. In the case of subtotal sternotomy, when a part of the manubrium or the lower sternal body are conserved, a rigid reconstruction could be unnecessary; further, these defects can be replaced by non-rigid prostheses (11).
Scarnecchia et al. demonstrated that in patients who underwent CW resection, stabilization in the critical area has an inverse correlation with acute respiratory complications (P<0.001) (25).
There are various types of rigid implants in clinical practice, including Matrix-RIB (DePuy Synthes, West Chester, PA, USA), STRATOS (Strasburg Thoracic Osteosyntheses System, MedXpert GmbH, Heitersheim, Germany), Ribfix Blu (Zimmer Biomet, Warsaw, IN, USA) and Sternalock (Zimmer Biomet), titanium mesh, polymethylmethacrylate, and personalized implants (13). There are no relevant studies comparing the advantages of these implants; generally, titanium plate and mesh polymethylmethacrylate are the most used. Personalized implants, such as three-dimensional (3D) printed implants, can be fabricated using multiple biomaterials and have the advantage of anatomically repairing the CW defect, with very interesting clinical applications (26). The process to design, produce, and implant these prostheses involves collaboration with a clinical engineer and is only allowed in clinical trials in USA and China.
Since the CW is a dynamic structure, some authors believe that the use of rigid materials increases the risk of rib fracture or prothesis migration with dermal or visceral erosion. The use of synthetic materials as sheets or mesh has the advantage of providing a stabilizing membrane in CW reconstruction especially in lateral defect; these can be modeled on the defect area, restore the integrity of CW with varying degrees of flexibility, and are generally less expensive than rigid prothesis (Figure 2) (27). Options include absorbable and non-absorbable materials. The most absorbable meshes used are Vycril® (Ethicon Inc., Sommerville, NJ, USA), and PDS® (Ethicon Inc.). Marlex mesh (Bard and Davol, Cranston, RI, USA), Prolene mesh (Ethicon Inc.), Gore-Tex®/e-PTFE (Gore, Flagstaff, AZ, USA), and Mersilene mesh (Ethicon Inc.) are the commonly used non-absorbable types of mesh for chest reconstruction. The choice of material falls to surgeon preference, institutional availability, and cost; there is no evidence favoring absolutely a particular type over another in terms of clinical outcomes. They are simple to use, can be adapted to CW defect, and fastened with continuous or interrupted sutures to the surrounding tissue (ribs, fascia, muscles).
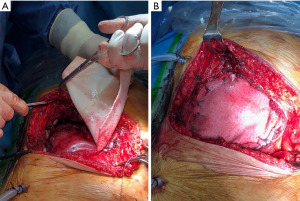
In recent years, bio-prosthetic meshes have been proposed in CW surgery. Conceived for use in abdominal hernia repair (28), bio-prosthetic meshes can be used to cover small or moderate size defects where rigidity is not essential. The meshes are derived from processed extracellular matrix from human or animal sources, and their properties include the ability to support tissue regeneration through revascularization and cell repopulation, biological integration, and resistance to infection with a good antibiotic penetration. These types of meshes include homograft derived from cadaveric human dermis (AlloDerm®; Tutogen Medical Inc., Alachua, FL, USA), and allograft such as porcine dermis (Permacol®; Medtronic, Minneapolis, MN, USA), bovine dermis, and bovine pericardium. The surgical technique is similar to that of synthetic mesh.
CW reconstruction is generally viewed as a procedure with two aspects: CW stabilization and soft tissue reconstruction. Satisfactory cosmesis is an important secondary goal that merits careful consideration. After the restitution of the CW integrity, soft tissue coverage can be provided by direct closure, pedicled myocutaneous flaps, or free flap and skin grafts; cooperation with plastic surgeons is essential.
Conclusions
Lung cancer with CW invasion represents a surgical disease in the majority of patients. The most important prognostic factors are free surgical margins and lymph node staging. Preoperative evaluation includes functional and oncological evaluation, whereby CT scan and MRI of the thorax are mandatory for the planning of surgical CW resection and subsequent reconstruction. In the last decades, a minimally invasive surgical approach was applied to en block CW resection, with good postoperative and oncological results. Many techniques could be used for CW reconstruction, including different types of rib plates and meshes, to restore the integrity of the chest with the aim to protect underlying organs and avoid abnormal lung ventilation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alessandro Gonfiotti) for the series “Chest Wall Surgery” published in Shanghai Chest. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-23-24/rc
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-23-24/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-23-24/coif). The series “Chest Wall Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Doddoli C, D'Journo B, Le Pimpec-Barthes F, et al. Lung cancer invading the chest wall: a plea for en-bloc resection but the need for new treatment strategies. Ann Thorac Surg 2005;80:2032-40. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Coleman FP. Primary Carcinoma of the Lung, with Invasion of the Ribs: Pneumonectomy and Simultaneous Block Resection of the Chest Wall. Ann Surg 1947;126:156-68. [Crossref] [PubMed]
- Bennett DT, Weyant MJ. Extended chest wall resection and reconstruction in the setting of lung cancer. Thorac Surg Clin 2014;24:383-90. [Crossref] [PubMed]
- Vilmann P, Frost Clementsen P, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2015;48:1-15. [Crossref] [PubMed]
- Eberhardt WE, De Ruysscher D, Weder W, et al. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol 2015;26:1573-88. [Crossref] [PubMed]
- Drake JA, Sullivan JL, Weksler B. Adjuvant chemotherapy improves survival in patients with completely resected T3N0 non-small cell lung cancer invading the chest wall. J Thorac Cardiovasc Surg 2018;155:1794-802. [Crossref] [PubMed]
- Ahmad U, Crabtree TD, Patel AP, et al. Adjuvant Chemotherapy Is Associated With Improved Survival in Locally Invasive Node Negative Non-Small Cell Lung Cancer. Ann Thorac Surg 2017;104:303-7. [Crossref] [PubMed]
- Lanuti M. Surgical Management of Lung Cancer Involving the Chest Wall. Thorac Surg Clin 2017;27:195-9. [Crossref] [PubMed]
- Kawaguchi K, Yokoi K, Niwa H, et al. Trimodality therapy for lung cancer with chest wall invasion: initial results of a phase II study. Ann Thorac Surg 2014;98:1184-91. [Crossref] [PubMed]
- Wang L, Yan X, Zhao J, et al. Expert consensus on resection of chest wall tumors and chest wall reconstruction. Transl Lung Cancer Res 2021;10:4057-83. [Crossref] [PubMed]
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]
- Filosso PL, Sandri A, Guerrera F, et al. Primary lung tumors invading the chest wall. J Thorac Dis 2016;8:S855-62. [Crossref] [PubMed]
- Facciolo F, Cardillo G, Lopergolo M, et al. Chest wall invasion in non-small cell lung carcinoma: a rationale for en bloc resection. J Thorac Cardiovasc Surg 2001;121:649-56. [Crossref] [PubMed]
- Hanagiri T, Shinohara S, Takenaka M, et al. Clinical Characteristics of Resected T3 Non-small Cell Lung Cancer Characterized by Parietal Pleural Invasion or Chest Wall Invasion. Indian J Surg 2014;76:354-8. [Crossref] [PubMed]
- Dal Agnol G, Oliveira R, Ugalde PA. Video-assisted thoracoscopic surgery lobectomy with chest wall resection. J Thorac Dis 2018;10:S2656-63. [Crossref] [PubMed]
- Ueda Y, Nakagawa T, Toyazaki T, et al. Rib resection using a pneumatic high-speed power drill system for lung cancer with chest wall invasion: our clinical experience. Surg Today 2017;47:476-80. [Crossref] [PubMed]
- Guido-Guerrero W, Bolaños-Cubillo A, González-Rivas D. Single-port video-assisted thoracic surgery (VATS)-advanced procedures & update. J Thorac Dis 2018;10:S1652-61. [Crossref] [PubMed]
- Widmann MD, Caccavale RJ, Bocage JP, et al. Video-assisted thoracic surgery resection of chest wall en bloc for lung carcinoma. Ann Thorac Surg 2000;70:2138-40. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Single-incision thoracoscopic right upper lobectomy with chest wall resection by posterior approach. Innovations (Phila) 2013;8:70-2. [Crossref] [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. Feasibility of hybrid thoracoscopic lobectomy and en-bloc chest wall resection. Eur J Cardiothorac Surg 2012;41:888-92. [Crossref] [PubMed]
- Jaus MO, Forcione A, Gonfiotti A, et al. Hybrid treatment of T3 chest wall lung cancer lobectomy. J Vis Surg 2018;4:32. [Crossref] [PubMed]
- Hennon MW, Dexter EU, Huang M, et al. Does Thoracoscopic Surgery Decrease the Morbidity of Combined Lung and Chest Wall Resection? Ann Thorac Surg 2015;99:1929-34; discussion 1934-5. [Crossref] [PubMed]
- Puma F, Vannucci J. Chest wall resection/reconstruction for tumors. In: Fischer JE, editor. Master Techniques in Surgery: Thoracic Surgery. Philadelphia: Wolters Kluwer; 2015:312-58.
- Scarnecchia E, Liparulo V, Capozzi R, et al. Chest wall resection and reconstruction for tumors: analysis of oncological and functional outcome. J Thorac Dis 2018;10:S1855-63. [Crossref] [PubMed]
- Goldsmith I. Chest Wall Reconstruction With 3D Printing: Anatomical and Functional Considerations. Innovations (Phila) 2022;17:191-200. [Crossref] [PubMed]
- Sandler G, Hayes-Jordan A. Chest wall reconstruction after tumor resection. Semin Pediatr Surg 2018;27:200-6. [Crossref] [PubMed]
- Baumann DP, Butler CE. Bioprosthetic mesh in abdominal wall reconstruction. Semin Plast Surg 2012;26:18-24. [Crossref] [PubMed]
Cite this article as: Mantovani S, Jaus MO. Lung cancer infiltrating the chest wall: a narrative review. Shanghai Chest 2023;7:32.


