
Management of chronic pleural tuberculosis and non-tuberculous empyema in the 21st century
Introduction
Pleural tuberculosis (TB) and non-TB empyema are distinct pathologies, each presenting unique challenges to healthcare providers. To fully grasp the complexities of these conditions, it is essential to explore their pathogenesis and pathophysiology.
The pathogenesis of pleural TB often begins with the reactivation of latent Mycobacterium tuberculosis infection (1). The bacteria spread from the lung parenchyma to the pleural space, eliciting an immune response that accumulates fluid and inflammatory cells in the pleura. This process is characterized by a delayed hypersensitivity reaction central to pleural effusion formation.
In contrast, non-TB empyema typically results from bacterial infections, such as pneumonia, that extend into the pleural space. The accumulation of pus in the pleural cavity is a response to this infection, leading to a more acute inflammatory reaction compared to pleural TB.
The global prevalence of pleural TB remains obscure. Still, estimates suggest that pleural TB accounts for approximately 5% of all TB cases in endemic areas (2), and therefore the second cause of extra-pulmonary TB (3). As a highly geographically distinct disease, the prevalence of the pleural form may be higher in disease-dense regions (4).
Special populations include people co-infected with the human immunodeficiency virus (HIV), who demonstrate increased susceptibility to TB manifestations, including the pleural form (5).
Other susceptible populations have migrant and refugee populations, often encountering considerable barriers to effective healthcare (6), potentially resulting in complications and augmented disease transmission (7).
As expected in most diseases, socioeconomic factors significantly impact treatment outcomes (8). Additionally, pleural TB has economic and sociocultural consequences (9), often impeding an individual’s capacity to work for an extended period and precipitating social ostracism due to disease-associated stigma (10).
Similar to TB, non-TB pleural empyema represents a significant public health problem (11,12), with a vast number of disparities that are influenced by factors including average life expectancy, pneumonia prevalence, accessibility to anti-pneumococcal vaccines, antibiotic resistance trends, availability of advanced treatments such as fibrinolytic, and prompt access to optimal medical and surgical care (13-16).
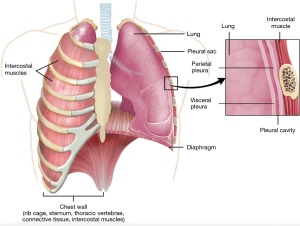
To facilitate understanding, we provide a detailed figure of pleural anatomy (Figure 1). This visual aid illustrates the thoracic structures involved in pleural diseases, serving as a reference point throughout this review.
History of pleural TB
Historically dominated by surgical interventions, TB has been considered by many “the mother of thoracic surgery” (17). From highly invasive thoracoplasties (18), the management of the disease transformed with the advent of anti-TB agents like streptomycin by the mid-20th century (19), shifting swiftly from surgical modalities.
The evolution of anti-TB agents [e.g., isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (EMB)] (20) advanced medical therapy as the primary treatment modality, reserving surgical interventions for cases presenting with complications like bronchopleural fistulas (21) or drug-resistant forms (22).
Now, in the early 21st century, the diagnosis of TB has been enhanced by advanced imaging modalities [e.g., computed tomography (CT) and magnetic resonance imaging (MRI)] (23) complemented by enhanced laboratory techniques (e.g., GeneXpert MTB/RIF assay), expediting an accurate diagnostic process (24-26).
History of non-TB empyema
Although the history of pleural empyema is said to be traced back to Aristotle 2,000 years ago (27), the first clinical reports of the disease date back to the 1800s by surgeons such as Bell, Playfair, Meyer, and Bulau (28).
Primarily managed with open drainage (29,30) or surgeries such as rib trephining (31), the introduction of tube thoracostomy as a minimally invasive alternative only became standard during World War II (32).
In the mid-20th century, the antibiotic revolution, spearheaded by agents like penicillin (33), substantially influenced empyema management by addressing its bacterial cause. Subsequently, decortication through thoracotomy emerged as a surgical mainstay for organized empyema cases (34).
A main technological advancement in the 21st century was the introduction of video-assisted thoracoscopic surgery (VATS), which offered similar results for the early stages of the disease, with reduced pain and gained better acceptance (30). Fibrinolytic agents [e.g., streptokinase, urokinase, deoxyribonuclease (DNase), and alteplase] also gained popularity, aiding drainage and empyema resolution without operations (35).
Clinical presentation and diagnosis
Understanding the clinical presentation and accurately diagnosing pleural TB and non-TB empyema are critical for effective management.
A comparative summary of both diseases has been provided in Table 1.
Table 1
| Criteria | Pleural TB | Non-TB empyema |
|---|---|---|
| Clinical presentation | Insidious onset, fever, night sweats, pleuritic chest pain | Acute onset, high fever, chest pain, dyspnea |
| Laboratory findings | Lymphocytic pleural effusion, elevated ADA levels | Neutrophilic pleural effusion, low pH and glucose levels |
| Imaging features | Pleural thickening and pleural effusion on ultrasound or CT scan | Loculated effusion on chest X-ray, or ultrasound, thickened pleura on, loculations, dense fluid or split pleura on CT scan |
| Microbiological tests | Positive Mycobacterium tuberculosis culture or PCR | Positive bacterial culture, often gram-positive cocci |
TB, tuberculosis; ADA, adenosine deaminase; CT, computed tomography; PCR, polymerase chain reaction.
Pleural TB primarily manifests as a chronic pleural effusion, often accompanied by an array of systemic symptoms: persistent cough, nocturnal hyperhidrosis, significant weight loss, and intermittent fever (36).
Direct isolation of Mycobacterium tuberculosis remains arduous due to prolonged cultivation periods and a diagnostic yield of only 63% (37).
Alternative diagnostic modalities encompass pleural fluid analysis of adenosine deaminase (ADA) levels with (cutoff >40 U/L) (38), which has sensitivity of 92% and a specificity of 90% and/or molecular-based detection via the GeneXpert MTB/RIF assay, which has a sensitivity of 98% and specificity of 98% (39) in TB empyema and 40–50% in conventional pleural TB (39). This reduced yield can be explained by the pleural effusions being mostly a hypersensitivity reaction.
Unlike pleural TB, non-TB empyema predominantly presents acutely and is characterized by fever, pleuritic chest pain, dyspnea, and a productive cough (40).
Etiologically, non-TB empyema is an infectious pleural effusion engendered not by Mycobacterium tuberculosis, but by a plethora of pathogens.
Classic bacterial causes are Streptococcus pneumoniae, Staphylococcus aureus, and various Gram-negative bacilli (41) and anaerobes such as Fusobacterium nucleatum and/or Streptococcus spp. (42).
Depending on the hospital and geographic country, specific causes vary based on local microbiology. Culture sensitivity also varies locally but typically ranges at around 46% for pleural fluid, 46.9% for tissue culture, and 62.7% for a combination of tissue and culture (43). Some more optimistic reports have described a yield of up to 92% (44).
Although the most common cause is pneumonia, there are other etiologies such as esophageal perforation, thoracic surgical interventions, or cervical infections (45).
Diagnostic criteria for non-TB empyema necessitate pleural fluid analysis showing pus, a positive fluid culture or gram stain, lactate dehydrogenase (LDH) levels surpassing 1,000 U/L, or a pH, typically below 7.2 (46).
To further clarify, In Table 2, we provide a summary of key distinguishing features between the two diseases.
Table 2
| Features | Pleural TB | Non-TB empyema |
|---|---|---|
| Onset | Gradual | Rapid |
| Symptoms | Mild fever, weight loss, chest pain | High fever, severe chest pain, dyspnea |
| Pleural fluid characteristics | Lymphocytic, high ADA | Neutrophilic, low pH, high LDH |
| Radiological signs | Unilateral effusion, pleural thickening | Loculated effusions, possible lung consolidation |
| Causative agent | Mycobacterium tuberculosis | Various bacteria, often community-acquired |
TB, tuberculosis; ADA, adenosine deaminase; LDH, lactate dehydrogenase.
We provide distinct clinical vignettes about these two diseases.
Case presentation 1
Mr. A, a 45-year-old male, presented with a 3-week history of low-grade fever, night sweats, and left-sided chest pain. He had a history of exposure to TB. He had decreased breath sounds over the left lower lung field on examination. Chest X-ray revealed left-sided pleural effusion. Pleural fluid analysis showed lymphocytic predominance and elevated ADA levels. A diagnosis of pleural TB was made based on these findings.
Learning point
This vignette illustrates the typical presentation and diagnostic approach for pleural TB, emphasizing the importance of history, physical examination, and targeted investigations.
Case presentation 2
Mrs. B, a 60-year-old female with a history of chronic obstructive pulmonary disease (COPD), presented to the emergency department with a 5-day history of high fever, sharp right-sided chest pain, and shortness of breath. She had a productive cough with greenish sputum. Her recent medical history included a severe bacterial pneumonia 10 days prior. On physical examination, she exhibited respiratory distress and decreased breath sounds over the right lower lung field. A chest X-ray revealed a right-sided loculated pleural effusion.
Pleural fluid analysis showed a neutrophilic predominance, low pH, and elevated LDH levels. Gram stain of the pleural fluid revealed gram-positive cocci. Based on these findings, a diagnosis of non-TB empyema was made.
Learning point
This vignette highlights the acute presentation and diagnostic criteria for non-TB empyema, underscoring the importance of considering past medical history, especially recent bacterial infections, in the diagnostic process. The case also emphasizes the role of pleural fluid analysis and imaging in confirming the diagnosis.
Management strategies
Managing pleural TB and non-TB empyema includes various therapeutic tools, requiring a balance of evidence-based practices and practical considerations.
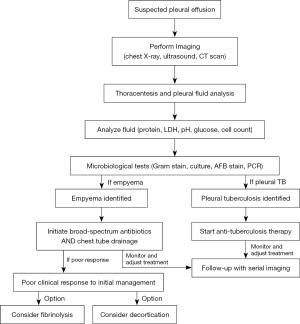
A treatment algorithm (Figure 2) outlines main steps from diagnosis to treatment completion, including first-line therapies, and surgical interventions.
The standard of care for pleural TB typically involves a 6–9-month course of anti-TB therapy, adhering to WHO guidelines. For non-TB empyema, the approach is antibiotic therapy tailored to culture results and drainage procedures, following American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) guidelines.
This section will explain the broader spectrum of available therapies, providing clarity on conventional practices and their rationale.
(I) Medical management of pleural TB
The cornerstone of pleural TB management is anchored in a combination regimen comprising INH, RIF, PZA, and EMB (47).
This therapeutic strategy is bifurcated into an initiation phase, where INH, RIF, PZA, and EMB are administered daily over 2 months, followed by a continuation phase of daily INH and RIF over 4 months (47).
Special considerations may necessitate extending the consolidation phase to 7 months, such as extensive TB cases (high burden of disease), hepatotoxicity, or concurrent HIV infection (47). Cases that require a change in the treatment regimen require an extension of the consolidation phase to at least 9 months.
Adjunctive interventions, such as prednisolone, may be contemplated in cases with pronounced pleural thickening or dyspnea (48). However, the evidence underscoring its efficacy remains low (49).
Periodic evaluations, sputum cultures, liver function tests, and serial chest radiographs are imperative to check therapeutic progress and identify potential adverse reactions (50). Antibiotic resistance is a significant public health problem, particularly in developing countries. Therefore, for all patients initiating treatment and in special situations where drug-resistant TB is suspected, drug susceptibility testing is mandatory (51).
HIV patients are required to begin simultaneous antiretroviral therapy (ART) once stable. TB treatment must always precede the ART treatment.
(II) Medical management of non-TB empyema
Pleural empyema’s medical management consists of broad-spectrum antibiotics, including anaerobic coverage (52). Antibiotic regimens can then be adjusted based on pleural fluid cultures and sensitivities and typically last 14 to 21 days (53). Conventionally, intravenous antibiotic is started and then transitioned or changed to oral formulations contingent on clinical improvement and microbial sensitivity profiles.
(III) Indications for chest tube placement
Therapeutic drainage of large effusions in both CPT and non-TB empyema is usually indicated.
Thoracentesis may allow diagnosis (54), allowing for fluid cultures, biochemical assessment, and cytologic analyses. Additionally, clinically, patients may experience pulmonary expansion, symptom improvement, and functional enhancement. Installation of a chest tube or small pigtail may be a better initial choice for continuous drainage, especially considering ultrasound-guided insertion (55). Optimal drainage can also potentiate the penetration of anti-TB medications into the pleural space (51).
Management involving chest tube placement, once pleural TB has been initially drained, remains a topic of debate, as such effusions might remit following concomitant antibiotic therapy (56).
While tube thoracostomy inherently bears risks—spanning infection, iatrogenic injury, and pain (57)—recent incorporation of small-bore chest tubes (58) and auxiliary imaging for placement have mitigated these risks, enhancing precision (59). Contemporary high-level evidence supports the safety and efficacy of smaller drains, dispelling some entrenched surgical myths (60).
Permanent chest tube placement can be a source of significant discomfort, making routine long-term placement generally inadvisable, except for chronic empyema’s in patients who are not surgical candidates and have not achieved source control with fibrinolytic therapy (61).
(IV) Fibrinolytics
Intrapleural use of fibrinolytics in non-TB empyema has traditionally been indicated in patients who are not good candidates for surgical treatment due to comorbidities or frailty (62). However, additional indications such as residual infections after decortication or even as a primary treatment instead of VATS surgery may be considered depending on the provider’s choice (63). Fibrinolytic superiority to surgery in adults has yet to be proven (64-66). Cost-effectiveness between the two techniques may be similar (67), with length of admission being shorter in surgical patients (66).
Several intrapleural medications have been historically used, such as streptokinase (68,69), urokinase (63), DNase (70), alteplase (71), and their combination (72). The MIST-2 trial favors a combination of DNase and alteplase as the most effective treatment strategy (72). Despite the evidence, it is still expected to have a high amount of heterogeneity regarding treatment choice (73), dosages (74), and frequency of administration (75).
Regarding the role of pleural TB, fibrinolytic therapy has no universally accepted indication (76,77), with the exception being pleural effusions with a high number of differential diagnoses or a concurrent non-TB empyema (35).
(V) Surgical and interventional management of pleural disorders
Pleural biopsy
Indications for pleural biopsy emerge when malignancies or TB constitute potential differential diagnoses. Tissue analysis allows for a high diagnostic accuracy (78) and may be done through needle biopsy (79), medical thoracoscopy (80,81), or VATS. If needed, VATS can be performed awake (82), and the procedure is associated with a prompt postoperative recovery and a high diagnostic yield (83). In non-TB empyema’s, pleural peel is sent to pathological analysis when the differential diagnosis is high for pleural malignancies.
Decortication
Decortication entails a surgical exploration of the thoracic cavity, coupled with the aspiration of pleural effusion and the surgical excision of fibrinous and inflammatory deposits from the visceral pleura to promote pulmonary re-expansion, clean the chest cavity and reduce bacterial load (84).
This surgical intervention is predominantly executed through minimally invasive methodologies, notably VATS (85). Robotic-assisted thoracic surgery (RATS) has also been utilized (86), yet there is no evidence to support it over the VATS technique.
VATS allows faster recovery, reduced pain, and reduced respiratory complications compared to thoracotomy (87). Still, the surgery does have well-described complications (88) such as bleeding needing a transfusion, air-leak, respiratory failure, postoperative myocardial infarction, acute kidney injury, mechanical ventilation, among others (89).
As for pleural TB, decortication was a widely used technique of mostly historic interest (90). It is currently only routinely performed if patients present with chronic pleural thickening despite aggressive and prolonged medical treatment (91).
Extrapleural pneumonectomy (EPP)
EPP was first described for treating pleural TB refractory to thoracoplasty (92). With the advances in medical treatment, this technique has fortunately been essentially extinct for this indication (93).
EPP has no role in the management of non-TB empyema’s.
Pleurodesis
There is no robust evidence advocating chemical pleurodesis to prevent the recurrence of pleural TB. Additionally, this intervention might render subsequent surgical access to the thoracic cavity more troublesome and may prove ineffective due to trapped lung (94).
Regarding non-TB empyema, chemical pleurodesis is a contraindication due to the potential augmentation of complications and suboptimal infection management (95).
(VI) Management of complications
In addressing the spectrum of empyema, it is crucial to highlight the challenging scenario of empyema with bronchopleural fistula. This condition, often overlooked, represents a clinical challenge, frequently arising from complex etiologies such as pulmonary mycosis, TB or non-TB mycobacterial infections, complications following pulmonary resection, or pulmonary abscesses.
Empyema with bronchopleural fistula is characterized by its refractory nature and the complexity of treatment, requiring antimicrobial therapy, and bronchoscopy and/or surgical treatment, which escapes the scope of this review.
(VII) Integration of treatment alternatives
In integrating treatment alternatives for non-TB empyema and pleural TB, it is imperative to consider the levels of evidence supporting each intervention and the data on cost-effectiveness to guide clinical decisions.
For non-TB empyema, the administration of broad-spectrum antibiotics, including anaerobic coverage, is strongly supported by high-quality evidence (Level I) (52), with the regimen’s adjustment based on culture sensitivities. The transition from intravenous to oral antibiotics is supported by evidence (Level II) (52) that reflects clinical improvement and microbial sensitivity profiles.
Chest tube placement, a key component of empyema management, is backed by substantial evidence (Level I) (52) for its indication in draining large effusions and, in the case of TB, facilitating the delivery of anti-TB medications into the pleural space. Using smaller-bore chest tubes and image-guided placement has decreased morbidity (Level II evidence) (55,60), enhancing patient comfort and reducing procedure-related risks.
Fibrinolytic therapy in non-TB empyema offers an alternative to surgical intervention, particularly for patients with significant comorbidities (Level II evidence) (65). While the cost-effectiveness of fibrinolytic compared to surgery is similar (Level III evidence) (67), the length of hospital stay tends to be shorter for patients undergoing surgery (Level II evidence) (67). Specific agents like DNase and alteplase are supported by trials such as MIST-2 (Level I evidence) (72).
Surgical management for empyema includes VATS and decortication, supported by high-level evidence (Level I and II) (88) for their efficacy in rapid recovery. Despite known complications, VATS is favored over open thoracotomy for its lower incidence of respiratory complications and postoperative pain (Level II evidence) (30).
The role of pleural TB management in surgery, particularly decortication, is reserved for cases of chronic pleural thickening after prolonged medical therapy, as suggested by lower-level evidence (Level III) (17). EPP, once a treatment for pleural TB, has become obsolete due to medical advancements (Level IV evidence) (92).
Chemical pleurodesis is not commonly advocated in pleural TB or non-TB empyema due to a lack of solid evidence and potential complications that can arise from the procedure (95).
Integrating these treatment alternatives must be supported by thoroughly understanding the evidence and cost-effectiveness. This ensures that each patient receives the most appropriate, evidence-based care tailored to their clinical context and the physician’s place of practice. This approach promotes medically and economically optimal outcomes, aligning healthcare interventions.
Discussion
This review, while comprehensive, has its limitations. The rapidly evolving nature of healthcare, especially in the context of the COVID-19 pandemic, means that some recent developments may be excluded. Additionally, the variability in healthcare access and quality worldwide may affect the generalizability of our work.
Both TB effusions and non-TB pleural empyema currently pose challenges for medical professionals. As advancements have been made in healthcare, these conditions have become less common in developed countries.
Increased migration and social conflicts may have created a surge in these diseases in populations living in vulnerable conditions without data to analyze their impact.
There remain significant knowledge gaps and controversies in the diagnosis and treatment of pleural TB and non-TB empyema. For instance, the optimal use of surgical interventions in pleural TB and the management of drug-resistant forms of non-TB empyema are areas of ongoing debate. These gaps highlight the need for further research, particularly in understanding the benefits of different therapeutic approaches.
Conclusions
In conclusion, this review thoroughly explores TB and non-TB pleural empyema, highlighting their historical context, clinical presentations, and current management strategies.
Key takeaway points
- A high index of suspicion is crucial for the timely diagnosis of pleural TB, and medical treatment is the cornerstone of management.
- Managing non-TB empyema requires a multidisciplinary approach tailored to the patient’s needs, including antibiotics, drainage, and potentially surgery or fibrinolytics.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Rahul Nayak) for the series “Management of Pleural Diseases in the 21st Century” published in Shanghai Chest. The article has undergone external peer review.
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-23-32/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-23-32/coif). The series “Management of Pleural Diseases in the 21st Century” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shaw JA, Diacon AH, Koegelenberg CFN. Tuberculous pleural effusion. Respirology 2019;24:962-71. [Crossref] [PubMed]
- Seibert AF, Haynes J Jr, Middleton R, et al. Tuberculous pleural effusion. Twenty-year experience. Chest 1991;99:883-6. [Crossref] [PubMed]
- Antonangelo L, Faria CS, Sales RK. Tuberculous pleural effusion: diagnosis & management. Expert Rev Respir Med 2019;13:747-59. [Crossref] [PubMed]
- Rolo M, González-Blanco B, Reyes CA, et al. Epidemiology and factors associated with Extra-pulmonary tuberculosis in a Low-prevalence area. J Clin Tuberc Other Mycobact Dis 2023;32:100377. [Crossref] [PubMed]
- Relkin F, Aranda CP, Garay SM, et al. Pleural tuberculosis and HIV infection. Chest 1994;105:1338-41. [Crossref] [PubMed]
- Meaza A, Tola HH, Eshetu K, et al. Tuberculosis among refugees and migrant populations: Systematic review. PLoS One 2022;17:e0268696. [Crossref] [PubMed]
- Proença R, Mattos Souza F, Lisboa Bastos M, et al. Active and latent tuberculosis in refugees and asylum seekers: a systematic review and meta-analysis. BMC Public Health 2020;20:838. [Crossref] [PubMed]
- Nidoi J, Muttamba W, Walusimbi S, et al. Impact of socio-economic factors on Tuberculosis treatment outcomes in north-eastern Uganda: a mixed methods study. BMC Public Health 2021;21:2167. [Crossref] [PubMed]
- Hargreaves JR, Boccia D, Evans CA, et al. The social determinants of tuberculosis: from evidence to action. Am J Public Health 2011;101:654-62. [Crossref] [PubMed]
- Cremers AL, de Laat MM, Kapata N, et al. Assessing the consequences of stigma for tuberculosis patients in urban Zambia. PLoS One 2015;10:e0119861. [Crossref] [PubMed]
- Finley C, Clifton J, Fitzgerald JM, et al. Empyema: an increasing concern in Canada. Can Respir J 2008;15:85-9. [Crossref] [PubMed]
- Grijalva CG, Zhu Y, Nuorti JP, et al. Emergence of parapneumonic empyema in the USA. Thorax 2011;66:663-8. [Crossref] [PubMed]
- Lehtomäki A, Nevalainen R, Ukkonen M, et al. Trends in the Incidence, Etiology, Treatment, and Outcomes of Pleural Infections in Adults Over a Decade in a Finnish University Hospital. Scand J Surg 2020;109:127-32. [Crossref] [PubMed]
- Khan JA, Lehtomäki AI, Toikkanen VJ, et al. Long-Term Prognosis and Causes of Death After Pleural Infections. Scand J Surg 2018;107:145-51. [Crossref] [PubMed]
- Nanayakkara B, Tai J, Lemberger J, et al. Outcomes of empyema in the elderly: A study from Australia's Capital Territory. Eur Respir J 2018;52:PA4685.
- Mitchell MA, Deschner E, Dhaliwal I, et al. Association of Patient Demographics and Comorbidities with Clinical Outcomes in Adults Hospitalized for Empyema. Ann Am Thorac Soc 2021;18:904-6. [Crossref] [PubMed]
- Molnar TF. Tuberculosis: mother of thoracic surgery then and now, past and prospectives: a review. J Thorac Dis 2018;10:S2628-42. [Crossref] [PubMed]
- Meyer HW. Early history of extrapleural thoracoplasty for pulmonary tuberculosis in New York City. J Thorac Surg 1941;10:691-6. [Crossref]
- Streptomycin treatment of pulmonary tuberculosis. Br Med J 1948;2:769-82. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Treatment for TB Disease. 2023. Accessed September 23, 2023. Available online: https://www.cdc.gov/tb/topic/treatment/tbdisease.htm
- Stewart AGA, Boyd SC. Tuberculous bronchopleural fistula. Respirol Case Rep 2021;9:e00740. [Crossref] [PubMed]
- Calligaro GL, Singh N, Pennel TC, et al. Outcomes of patients undergoing lung resection for drug-resistant TB and the prognostic significance of pre-operative positron emission tomography/computed tomography (PET/CT) in predicting treatment failure. EClinicalMedicine 2023;55:101728. [Crossref] [PubMed]
- Skoura E, Zumla A, Bomanji J. Imaging in tuberculosis. Int J Infect Dis 2015;32:87-93. [Crossref] [PubMed]
- Habte D, Melese M, Hiruy N, et al. The additional yield of GeneXpert MTB/RIF test in the diagnosis of pulmonary tuberculosis among household contacts of smear positive TB cases. Int J Infect Dis 2016;49:179-84. [Crossref] [PubMed]
- Creswell J, Qin ZZ, Gurung R, et al. The performance and yield of tuberculosis testing algorithms using microscopy, chest x-ray, and Xpert MTB/RIF. J Clin Tuberc Other Mycobact Dis 2019;14:1-6. [Crossref] [PubMed]
- Andama A, Jaganath D, Crowder R, et al. Accuracy and incremental yield of urine Xpert MTB/RIF Ultra versus Determine TB-LAM for diagnosis of pulmonary tuberculosis. Diagn Microbiol Infect Dis 2020;96:114892. [Crossref] [PubMed]
- Brims FJ, Lansley SM, Waterer GW, et al. Empyema thoracis: new insights into an old disease. Eur Respir Rev 2010;19:220-8. [Crossref] [PubMed]
- Walcott-Sapp S, Sukumar M. A History of Thoracic Drainage: From Ancient Greeks to Wound Sucking Drummers to Digital Monitoring. 2022. doi: 10.25373/ctsnet.21291078.v1.
- Meyer JA. Gotthard Bülau and closed water-seal drainage for empyema, 1875-1891. Ann Thorac Surg 1989;48:597-9. [Crossref] [PubMed]
- Chan DT, Sihoe AD, Chan S, et al. Surgical treatment for empyema thoracis: is video-assisted thoracic surgery "better" than thoracotomy? Ann Thorac Surg 2007;84:225-31. [Crossref] [PubMed]
- Robinson S. Acute Thoracic Empyema. Avoidance of Chronic Empyema; Rib Trephining for Suction Drainage. The Boston Medical and Surgical Journal 1910;163:561-70. [Crossref]
- Monaghan SF, Swan KG. Tube thoracostomy: the struggle to the "standard of care". Ann Thorac Surg 2008;86:2019-22. [Crossref] [PubMed]
- Gaynes R. The discovery of penicillin—new insights after more than 75 years of clinical use. Emerg Infect Dis 2017;23:849-53. [Crossref]
- Carey JA, Hamilton JR, Spencer DA, et al. Empyema thoracis: a role for open thoracotomy and decortication. Arch Dis Child 1998;79:510-3. [Crossref] [PubMed]
- Altmann ES, Crossingham I, Wilson S, et al. Intra-pleural fibrinolytic therapy versus placebo, or a different fibrinolytic agent, in the treatment of adult parapneumonic effusions and empyema. Cochrane Database Syst Rev 2019;2019:CD002312. [Crossref] [PubMed]
- Berger HW, Mejia E. Tuberculous pleurisy. Chest 1973;63:88-92. [Crossref] [PubMed]
- Ruan SY, Chuang YC, Wang JY, et al. Revisiting tuberculous pleurisy: pleural fluid characteristics and diagnostic yield of mycobacterial culture in an endemic area. Thorax 2012;67:822-7. [Crossref] [PubMed]
- Jiménez Castro D, Díaz Nuevo G, Pérez-Rodríguez E, et al. Diagnostic value of adenosine deaminase in nontuberculous lymphocytic pleural effusions. Eur Respir J 2003;21:220-4. [Crossref] [PubMed]
- Sehgal IS, Dhooria S, Aggarwal AN, et al. Diagnostic Performance of Xpert MTB/RIF in Tuberculous Pleural Effusion: Systematic Review and Meta-analysis. J Clin Microbiol 2016;54:1133-6. [Crossref] [PubMed]
- BMJ Best Practice. Empyema. Accessed August 30, 2022. Available online: https://bestpractice.bmj.com/topics/en-us/1008
- Mandal AK, Thadepalli H. Treatment of spontaneous bacterial empyema thoracis. J Thorac Cardiovasc Surg 1987;94:414-8. [Crossref] [PubMed]
- Dyrhovden R, Nygaard RM, Patel R, et al. The bacterial aetiology of pleural empyema. A descriptive and comparative metagenomic study. Clin Microbiol Infect 2019;25:981-6. [Crossref] [PubMed]
- Cheng YF, Cheng CY, Huang CL, et al. Pleural Peels Tissue Culture plus Pleural Fluid Culture Help to Improve Culture Rate for Empyema. J Clin Med 2022;11:1882. [Crossref] [PubMed]
- Ferguson AD, Prescott RJ, Selkon JB, et al. The clinical course and management of thoracic empyema. QJM 1996;89:285-9. [Crossref] [PubMed]
- Garvia V, Paul M. Empyema. In: StatPearls. Treasure Island: StatPearls Publishing; 2023.
- Botana Rial M, Pérez Pallarés J, Cases Viedma E, et al. Diagnosis and Treatment of Pleural Effusion. Recommendations of the Spanish Society of Pulmonology and Thoracic Surgery. Update 2022. Arch Bronconeumol 2023;59:27-35. [Crossref] [PubMed]
- Nahid P, Dorman SE, Alipanah N, et al. Executive Summary: Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin Infect Dis 2016;63:853-67. [Crossref] [PubMed]
- Sun F, Li L, Liao X, et al. Adjunctive use of prednisolone in the treatment of free-flowing tuberculous pleural effusion: A retrospective cohort study. Respir Med 2018;139:86-90. [Crossref] [PubMed]
- Wyser C, Walzl G, Smedema JP, et al. Corticosteroids in the treatment of tuberculous pleurisy. A double-blind, placebo-controlled, randomized study. Chest 1996;110:333-8. [Crossref] [PubMed]
- Monitoring during treatment. In: Treatment of Tuberculosis: Guidelines. 4th ed. Geneva: World Health Organization; 2010.
- Zhai K, Lu Y, Shi HZ. Tuberculous pleural effusion. J Thorac Dis 2016;8:E486-94. [Crossref] [PubMed]
- Guideline Central. AATS Management of Empyema Guideline Summary. Accessed September 23, 2023. Available online: https://www.guidelinecentral.com/guideline/10539
- Hassan M, Gad-Allah M, El-Shaarawy B, et al. The Short versus Long Antibiotic Course for Pleural Infection Management (SLIM) randomised controlled open-label trial. ERJ Open Res 2023;9:00635-2022. [Crossref] [PubMed]
- Wiederhold BD, Amr O, Modi P, et al. Thoracentesis. In: StatPearls. Treasure Island: StatPearls Publishing; 2023.
- Liu YH, Lin YC, Liang SJ, et al. Ultrasound-guided pigtail catheters for drainage of various pleural diseases. Am J Emerg Med 2010;28:915-21. [Crossref] [PubMed]
- Lai YF, Chao TY, Wang YH, et al. Pigtail drainage in the treatment of tuberculous pleural effusions: a randomised study. Thorax 2003;58:149-51. [Crossref] [PubMed]
- Peris A, Tutino L, Cianchi G, et al. Ultrasound Guidance for Pleural-Catheter Placement. N Engl J Med 2018;378:e19. [Crossref] [PubMed]
- Mehra S, Heraganahally S, Sajkov D, et al. The effectiveness of small-bore intercostal catheters versus large-bore chest tubes in the management of pleural disease with the systematic review of literature. Lung India 2020;37:198-203. [Crossref] [PubMed]
- Taylor LA, Vitto MJ, Joyce M, et al. Ultrasound-guided thoracostomy site identification in healthy volunteers. Crit Ultrasound J 2018;10:28. [Crossref] [PubMed]
- Kulvatunyou N, Bauman ZM, Zein Edine SB, et al. The small (14 Fr) percutaneous catheter (P-CAT) versus large (28-32 Fr) open chest tube for traumatic hemothorax: A multicenter randomized clinical trial. J Trauma Acute Care Surg 2021;91:809-13. [Crossref] [PubMed]
- Krumm IR, Gesthalter YB. Use of tunneled pleural catheters in chronic empyema: Two case reports and brief review of the literature. Respir Med Case Rep 2022;40:101754. [Crossref] [PubMed]
- Idell S, Rahman NM. Intrapleural Fibrinolytic Therapy for Empyema and Pleural Loculation: Knowns and Unknowns. Ann Am Thorac Soc 2018;15:515-7. [Crossref] [PubMed]
- Lee S, Lee H, Lee DH, et al. Fibrinolysis with Lower Dose Urokinase in Patients with Complicated Parapneumonic Effusion. Tuberc Respir Dis (Seoul) 2021;84:134-9. [Crossref] [PubMed]
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017;3:CD010651. [Crossref] [PubMed]
- St Peter SD, Tsao K, Spilde TL, et al. Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: a prospective, randomized trial. J Pediatr Surg 2009;44:106-11; discussion 111. [Crossref] [PubMed]
- Petrakis IE, Kogerakis NE, Drositis IE, et al. Video-assisted thoracoscopic surgery for thoracic empyema: primarily, or after fibrinolytic therapy failure? Am J Surg 2004;187:471-4. [Crossref] [PubMed]
- Shipe ME, Maiga AW, Deppen SA, et al. Cost-Effectiveness Analysis of Fibrinolysis vs Thoracoscopic Decortication for Early Empyema. Ann Thorac Surg 2021;112:1632-8. [Crossref] [PubMed]
- Bouros D, Antoniou KM, Light RW. Intrapleural streptokinase for pleural infection. BMJ 2006;332:133-4. [Crossref] [PubMed]
- Davies RJ, Traill ZC, Gleeson FV. Randomised controlled trial of intrapleural streptokinase in community acquired pleural infection. Thorax 1997;52:416-21. [Crossref] [PubMed]
- Chong WH, Hu K, Saha BK, et al. Comparing the outcomes of intrapleural fibrinolytic and DNase therapy versus intrapleural fibrinolytic or DNase therapy: A systematic review and meta-analysis. Pulm Pharmacol Ther 2021;71:102081. [Crossref] [PubMed]
- Abdul Hamid MF, Hasbullah AHH, Mohamad Jailaini MF, et al. Retrospective review comparing intrapleural fibrinolytic therapy (alteplase) and surgical intervention in complex pleural effusion. BMC Pulm Med 2022;22:439. [Crossref] [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. [Crossref] [PubMed]
- Saxena K, Maturu VN. A Comparative Study of the Safety and Efficacy of Intrapleural Fibrinolysis With Streptokinase and Urokinase in the Management of Loculated Pleural Effusions. Cureus 2022;14:e26271. [Crossref] [PubMed]
- Sampsonas F, Lagadinou M, Mpouga M, et al. Use and effectiveness of intrapleural fibrinolytics in complicated parapneumonic effusions and empyemas. Eur Respir J 2022;60:3934.
- Kheir F, Cheng G, Rivera E, et al. Concurrent Versus Sequential Intrapleural Instillation of Tissue Plasminogen Activator and Deoxyribonuclease for Pleural Infection. J Bronchology Interv Pulmonol 2018;25:125-31. [Crossref] [PubMed]
- Chung CL, Chen CH, Yeh CY, et al. Early effective drainage in the treatment of loculated tuberculous pleurisy. Eur Respir J 2008;31:1261-7. [Crossref] [PubMed]
- Cases Viedma E, Lorenzo Dus MJ, González-Molina A, et al. A study of loculated tuberculous pleural effusions treated with intrapleural urokinase. Respir Med 2006;100:2037-42. [Crossref] [PubMed]
- Lo Cascio CM, Kaul V, Dhooria S, et al. Diagnosis of tuberculous pleural effusions: A review. Respir Med 2021;188:106607. [Crossref] [PubMed]
- Light RW. Establishing the diagnosis of tuberculous pleuritis. Arch Intern Med 1998;158:1967-8. [Crossref] [PubMed]
- Fu Z, Zhuang X, He Y, et al. Improved diagnosis of tuberculous pleural effusion by combining medical thoracoscopy with Interferon-Gamma Release Assay and adenosine deaminase activity. Food Sci Technol 2020;42:e38020. [Crossref]
- McDonald CM, Pierre C, de Perrot M, et al. Efficacy and Cost of Awake Thoracoscopy and Video-Assisted Thoracoscopic Surgery in the Undiagnosed Pleural Effusion. Ann Thorac Surg 2018;106:361-7. [Crossref] [PubMed]
- Gokce M, Altinsoy B, Piskin O, et al. Uniportal VATS pleural biopsy in the diagnosis of exudative pleural effusion: awake or intubated? J Cardiothorac Surg 2021;16:95. [Crossref] [PubMed]
- Medford AR, Awan YM, Marchbank A, et al. Diagnostic and therapeutic performance of video-assisted thoracoscopic surgery (VATS) in investigation and management of pleural exudates. Ann R Coll Surg Engl 2008;90:597-600. [Crossref] [PubMed]
- Kumar A, Anand S. Lung Decortication. In: StatPearls. Treasure Island: StatPearls Publishing; 2022.
- Subotic D, Lardinois D, Hojski A. Minimally invasive thoracic surgery for empyema. Breathe (Sheff) 2018;14:302-10. [Crossref] [PubMed]
- Khan AZ, Khanna S, Agarwal N, et al. Robotic thoracic surgery in inflammatory and infective diseases. Ann Cardiothorac Surg 2019;8:241-9. [Crossref] [PubMed]
- Shen KR, Bribriesco A, Crabtree T, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg 2017;153:e129-46. [Crossref] [PubMed]
- Towe CW, Carr SR, Donahue JM, et al. Morbidity and 30-day mortality after decortication for parapneumonic empyema and pleural effusion among patients in the Society of Thoracic Surgeons' General Thoracic Surgery Database. J Thorac Cardiovasc Surg 2019;157:1288-97.e4. [Crossref] [PubMed]
- Gorman J, Funk D, Srinathan S, et al. Perioperative implications of thoracic decortications: a retrospective cohort study. Can J Anaesth 2017;64:845-53. [Crossref] [PubMed]
- Savage T, Fleming HA. Decortication of the lung in tuberculosis disease; a study of 43 cases. Thorax 1955;10:293-308. [Crossref] [PubMed]
- Chen B, Zhang J, Ye Z, et al. Outcomes of Video-Assisted Thoracic Surgical Decortication in 274 Patients with Tuberculous Empyema. Ann Thorac Cardiovasc Surg 2015;21:223-8. [Crossref] [PubMed]
- SAROT IA. Extrapleural pneumonectomy and pleurectomy in pulmonary tuberculosis. Thorax 1949;4:173-223. [Crossref] [PubMed]
- Duranti L, Pardolesi A, Bertolaccini L, et al. Extra-pleural pneumonectomy. J Thorac Dis 2019;11:1022-30. [Crossref] [PubMed]
- Mierzejewski M, Korczynski P, Krenke R, et al. Chemical pleurodesis - a review of mechanisms involved in pleural space obliteration. Respir Res 2019;20:247. [Crossref] [PubMed]
- D'Ambrosio PD, de Araújo PHXN, Junior ER, et al. Risk factors related to pleural empyema after talc slurry pleurodesis. Clinics (Sao Paulo) 2022;77:100098. [Crossref] [PubMed]
Cite this article as: Pérez P, Bello A. Management of chronic pleural tuberculosis and non-tuberculous empyema in the 21st century. Shanghai Chest 2024;8:3.


