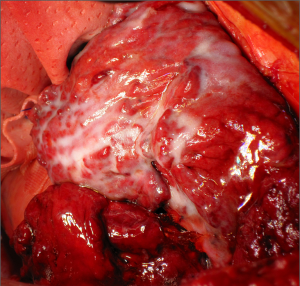
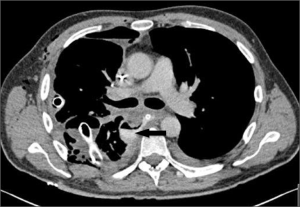
Pleurectomy/decortication for malignant pleural mesothelioma
Introduction
Malignant pleural mesothelioma (MPM) is a relatively rare malignancy arising from the mesothelial cells lining the pleural cavity with an increasing annual incidence in UK of over 2,000 cases per year. There is a clear association between occupational or environmental asbestos exposure and the development of MPM. There are two distinct histological subtypes of MPM: epithelioid (50–70%) and sarcomatoid (10–20%), with an intermediate classification of biphasic which may be epithelioid or sarcomatoid predominant. The epithelioid cell type is the most common and has a better prognosis. The standard treatment is platinum-based/pemetrexed chemotherapy and in some patient additional surgery may have a survival benefit. MPM is associated. Additional hemithorax irradiation may be used for local control. Recent reports suggested that patients undergoing surgery experienced a longer median survival compared with those without surgery (1). Concerns over the application of extrapleural pneumonectomy to an ageing population with reduced cardiorespiratory reserve and high treatment associated morbidity and mortality have led to a shift in opinion towards lung sparing surgery (2). Extended pleurectomy/decortication (EPD) is an operation intended to achieve removal of all visible tumour (macroscopic complete resection of the disease) without sacrificing the lung. It involves the resection of all parietal and visceral pleural tumours with associated resection of the diaphragm and/or pericardium if necessary (3). The IASLC Mesothelioma Domain suggested using the term “extended” rather than “radical” because of inability to eradicate residual microscopic disease. Radical term implies a completeness of resection with added therapeutic benefit and there was insufficient evidence that resection of the pericardium and diaphragm provides either (4).
Clinical setting
In order to establish the role of EPD within multimodality therapy we encourage surgery as part of the MARS2 trial: a randomised trial comparing (extended) pleurectomy decortication versus no pleurectomy decortication in the multimodality management of patients with MPM. This phase III study, which is on-going, needs to recruit over 300 patients and will report in 3–5 years. In the trial protocol all patients receive induction chemotherapy but EPD can be performed in chemonaive patients and as a salvage procedure for disease progression after first line chemotherapy.
Preoperative requirements
Operability
The patient must have sufficient cardiorespiratory reserve to tolerate the loss of the diaphragm but this may be countered by release of an entrapped lobe. In general if the patient would be considered fit enough to tolerate a lobectomy then they can proceed to EPD (5). In cases of doubt a differential lung perfusion scan may be helpful particularly where accurate assessment of pulmonary function may be difficult due to pleural effusion or entrapment.
Preoperative imaging of the chest and upper abdomen with recent staging CT-scan is indicated to establish the suitability of the patient for the surgery and planning the surgical strategy. CT-scan is important to identify the entity of invasion of the diaphragmatic muscle which occurs relatively early in the natural history of this disease (6), and to exclude invasion of major vascular structures, vertebrae or multifocal chest wall (cT4) or extra-thoracic lymph node metastases (cN2) disease (8th TNM revision). PET may be performed to exclude distant metastatic disease if there are equivocal appearances on CT-scan. If chest wall or neurovascular invasion is suspected, MRI may be helpful in preoperative planning, particularly in the apex of the pleural cavity.
Patients with clinical stage IV showing evidence of diffuse disease or metastatic disease should be excluded from surgery.
Operative technique
Preparation
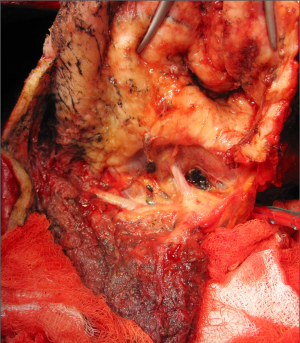
Following placement of an epidural catheter and induction of general anaesthesia a double-lumen endotracheal tube is used to facilitate single-lung ventilation. An arterial line and central venous line are inserted for invasive pressure monitoring. A 28Fr oesophageal bougie is used to identify the oesophagus during surgery to facilitate dissection. The patient is placed in the lateral decubitus position and to help the exposure the operative table is placed in a break position or a bean bag is placed to expand the rib cage and elevate the mediastinum (Figure 1).
Exposure
The incision for an extended posterolateral thoracotomy starts in front of the anterior axillary line, extending down toward the costal margin over the 9th rib and posteriorly beyond the tip of the scapula. The incision curves one or two finger breadths under the tip of the scapula and extends vertically on a line halfway between the posterior midline over the vertebral column and the medial edge of the scapula. The incision can be extended to widely excise all previous biopsy sites where there is potential for tumour progression. These biopsy sites are sent for histological examination since positive findings are a negative prognostic factor. Electrocautery is used for haemostasis and musculo-fascial dissection. The subcutaneous layer is divided down to the superior musculo-fascial plane. The dissection continues dividing, in the same plane, posteriorly the trapezius muscle and anteriorly the latissimus-dorsi muscle. Next, the inferior musculo-fascial plane is dissected dividing the lower portion of the rhomboid muscle and the fascia immediately posterior to the serratus muscle. The serratus muscle is spared and retracted anteriorly. The chest is entered via the 6th intercostal space, but if there is suggestion of close tumour apposition to the subclavian vessels or the inferior vena cava then the chest is entered via a double level 4th and 8th intercostal space. The periosteum is elevated off the rib superiorly and inferiorly. A periosteal elevator is used to separate the ribs from the surrounding soft tissue. A rib cutter is used to divide the lower rib anteriorly to the costovertebral angle to improve exposure. A rib spreader is placed to gain the initial room to start the dissection between the endothoracic fascia and the parietal pleura. Once an area large enough for a finger is created, blunt dissection of the tumour in the extrapleural plane can be commenced by digital manipulation.
Dissection
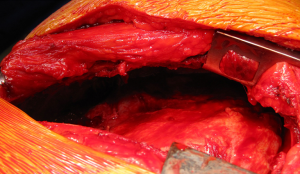
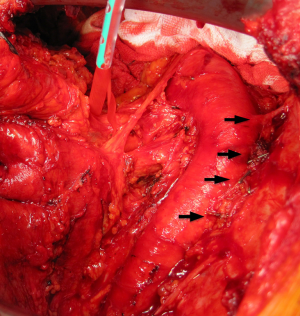
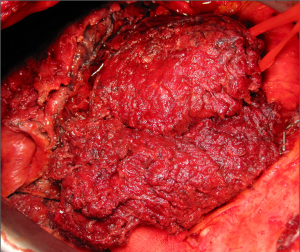
The dissection of the extrapleural plane is progressively developed first toward the apex with a posterior and anterior direction (Figure 2) then inferiorly toward the diaphragmatic surface. Both blunt and sharp dissections are used with electrocautery reserved for areas of dense adhesions. As the dissection moves anteriorly, the internal mammary vessels should be freed using sharp dissection and the internal mammary lymph nodal chain with surrounding adipose tissue should be removed. As the area of dissection increases, previous areas of dissection are packed with laparotomy pads for haemostasis. As the dissection progresses toward the apex great care must be used dissecting around the subclavian vessels. Once the superior portion of the lung is mobilised from the chest wall, intercostal arteries on both sides should be identified and tributary arteries may need to be ligated (Figure 3). From here blunt and sharp extrapleural plane dissection can be continued down towards the diaphragm to confirm resectability and assess diaphragmatic involvement before commencing dissection around the hilum and visceral surface.
Returning to the hilum, the dissection proceeds toward the mediastinum. On the right side: the azygos vein and superior vena cava must be approached and gently dissected with care; if the azygos vein is invaded and dissection is impossible, it may need to be divided. The right main stem bronchus is identified and exposed. The dissection of the oesophagus is begun at this level, then it is proceeded inferiorly along the oesophagus using sharp and blunt dissection; the bougie helps in palpating and identifying the oesophagus. It is important at this point is to identify and try to preserve the thoracic duct to avoid postoperative chylothorax. Then dissection continues on the posterior aspect of the pericardium where the vagus should be identified and protected and a subcarinal lymphadenectomy performed. On the left side: the dissection of the aorta starts using sharp and blunt dissection to enter the extrapleural plane over the vessel. Caution should be taken never to dissect behind the aorta. The dissection is continued medially along the aortic arch to the origin of the left subclavian and left carotid arteries, here the vagus nerve and the recurrent laryngeal branch should be identified and preserved. Then the dissection is continued distally along the descending aorta, the intercostal arteries are identified and tributary arteries may need to be ligated. Along the oesophagus superiorly to the aortic arch and inferiorly to hiatus is identified and exposed. Returning to hilum the left main bronchus is identified and exposed and subcarinal lymph node dissection is performed.
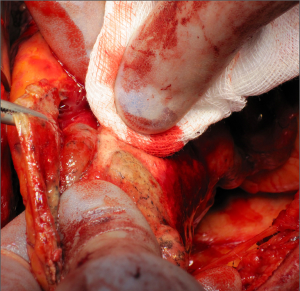
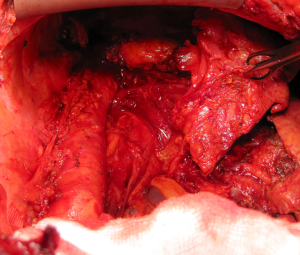
Once the parietal pleura have been freed in all areas attention is shifted toward the decortication of the lung by separating the visceral pleura from the underlying lung parenchyma. A sharp dissection is performed at the level of the visceral pleural reflection which is adherent to the tumor, on the main bronchus. The pleural reflection is undermined until a space between the visceral pleura and the lung parenchyma is created to allow digital insertion and blunt dissection. Where anatomical limitations do not allow using this approach; an alternative approach is to use a scalpel to make an incision in the tumour overlying the area of the oblique fissure down to lung parenchyma. The plane created between the visceral pleura, which is adherent to the tumour, and the lung is carefully developed with digital dissection by pushing along the rigid bronchus until a flap is raised. It is possible to mobilise a wide area of pleura from the underlying lung parenchyma working either side of the oblique fissure. Once a layer has been developed, it can be incised with electrocautery. At this point, each side of the flap is grasped (Figure 4) and the visceral pleurectomy continued using a gentle blunt dissection with a peanut sponge first and then with gentle digital sweeping motion of a small swab; sweeping should be exercised against the pleural sheet rather than the delicate underlying lung parenchyma (Figure 5). The plane of visceral dissection is continued superiorly towards the apex and across to the mediastinum then inferiorly to the interlobar fissure. It is important to achieve macroscopic tumour clearance around the interlobar structures by sharp dissection together with lymphadenectomy (Figure 6). Usually the upper lobe is cleared first then attention switches to the lower lobe. Visceral pleurectomy is continued down to the diaphragmatic surface. Direct parenchymal invasion by tumour may be encountered at the periphery of the lower lobe and less frequently in the upper and middle lobes. It is not necessary to perform more than a peripheral wedge resection using linear staplers. Once the lung parenchyma is completely decorticated with clear interlobar fissures and free from the hilar pleural reflection (Figure 7), the lung is pulled out of the tumour capsule and can be packed into the apex of the chest covered in laparotomy packs whilst attention switches to the pericardium and diaphragm.
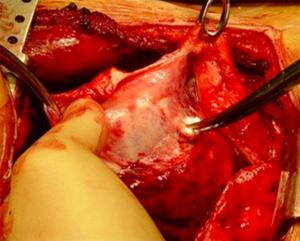
Very infrequently and only in very early stage of disease the pleural sheet might be bluntly dissected free from the pericardium and/or from the diaphragmatic muscle tissue preserving them. More frequently there is a substantial diaphragmatic and pericardial tumour invasion. It may be possible to remove the pleural tumour sheet en bloc with the pericardium and diaphragm but it is often easier to remove them separately.
Pericardiectomy
The pericardium is resected using sharp dissection. The incision starts close to the inferior pulmonary vein and is extended down to the diaphragm and across towards the midline. The pericardiophrenic connection is maintained. Digital protection of the heart is advised during pericardiotomy.
Phrenectomy
The diaphragm is resected bluntly by avulsing off the chest wall. The diaphragmatic muscle attachments to the chest wall are manually separated with a fingertip. This is performed from medial to lateral, preserving the peritoneum if possible and a rim of tumour-free muscle (Figure 8). On the left it is important to preserve a bridge of crural muscle over the hiatus to reduce the postoperative risk of hiatus hernia. On the right side at the oesophageal hiatus the inferior vena cava must be identified and carefully dissected out the inferior vena cava to ensure macroscopic clear margins since the tumour often wraps around the vessel. It may be necessary to skeletonise the IVC and ligate venous tributaries. The last step in the removal of the tumour is to complete the phrenectomy with the pericardium attached from medial to lateral in the right and lateral to medial on the left.
Prosthetic reconstruction
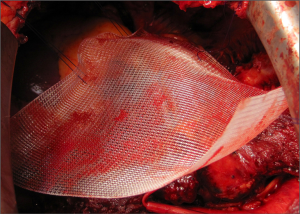
After resection of the diaphragm, care must be taken to repair firstly the pericardium to support the heart in a sling-like fashion without causing constriction and with a gap under the patch to prevent the risk of cardiac tamponade. The pericardium is reconstructed using preferably an absorbable material i.e., Vicryl mesh. Five or six evenly spaced interrupted non-absorbable sutures (i.e., Prolene-0) are passed through the remaining rim of pericardium to stabilise the Vicryl patch (Figure 9). The inferior of these can be used to anchor the diaphragm patch also.
The diaphragm is replaced with a bioprosthetic patch (i.e., Permacol) preferentially since persistent foreign material can act as a nidus for chronic infection in the presence of pleural sepsis. The patch is inserted with interrupted non-absorbable sutures (i.e., Prolene-0) into the peripheral rim of diaphragmatic muscle (Figure 10). In the most early cases it may be impossible to leave a significant peripheral sewing rim anteriorly. In most cases this anterior edge of the patch is secured with pericostal sutures tied on the exterior surface. The patch must be pulled taut to maximise expansion of the residual lung and prevent paradoxical movement of the neohemidiaphragm during inspiration. When postoperative radical hemithorax radiotherapy is planned the prosthesis should be inserted as low as possible, however, this is not routine after EPD and a higher insertion of the patch can aid in controlling the postoperative air leak from the lower lobe.
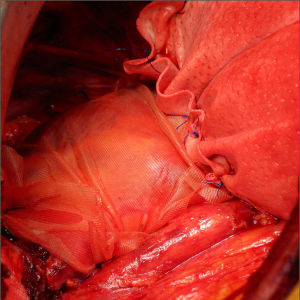
Following reconstruction and prior to closure it is imperative to perform a systematic lymph node dissection not only of the mediastinal but also the mammary, pericardial and diaphragmatic nodes. Enlarged intercostal nodes should also be removed.
Completion
Attention is now turned to obtaining haemostasis performed with electrocautery and surgical metal clips. In consideration of a large postoperative air leak from the denuded lung parenchyma it is imperative to adequately drain the cavity with three large bore drains which we usually place on low suction. Three 32-French chest tubes are inserted: one anterior-apical, one posterior-apical, and one angled basal. The angled tube is generally placed through the most posterior incision. At the end of procedure we perform lavage of the chest cavity with a solution of hyperthermic (40–42°) saline with 10% of Povidone. The iodine lavage prior to closure has been proposed as a useful adjunct that may have anti-tumour properties and may help to sterilise the cavity. The use of a parietal pleural haemostat is also advised. Similarly, the use of a visceral pleural aerostatic agent can reduce the inevitable postoperative air leak (Figure 11). There are many alternatives but an aerosolized administration reduces operative time.
Potential postoperative complications/management
Chylothorax−
The areas where the thoracic duct is at risk of iatrogenic damage are just above the aortic arch on the left and low down in the mediastinum on the right. The damage may not be apparent at the operation and may not manifest itself for several days.
The chylous leak can be managed conservatively by restricting oral intake and parenteral nutrition but this rarely succeeds. Early video assisted thoracoscopic clipping of the damaged duct is recommended and location of the leak is facilitated by oral administration of a high fat meal of 20 mL of cream 30 min prior to surgery. The leakage of chylous fluid is dramatically increased and easily visualised.
Oesophageal perforation–
A rare but important complication is a delayed oesophageal perforation. This may be more common in more advanced tumours particularly if the serosal layer of the organ is disrupted in dissection of the pleural sheet. Warning signs include sudden onset chest pain, tachyarrhythmia and subcutaneous emphysema. When detected within 24 hours operative repair is advised using buttressing with an intercostal muscle flap (Figure 12).
Diaphragmatic patch dehiscence–
This rare occurrence should be suspected by an abnormal chest radiograph showing elevation of the diaphragmatic patch and in particular a change from the immediate postoperative image. It may be suspected by the finding of an acute tachyarrhythmia in the immediate postoperative period.
Nasogastric suction (every 4 hour) is imperative in the first 24 hr after surgery to prevent acute gastric dilatation which may lead to patch disruption.
Persistent air leak—leading to pleural sepsis
The necessity to remove all the visceral inevitably leads to a prolonged postoperative air leak. There is no possibility to prevent this complication but the use of tissue glues on the raw lung surface can reduce the leak. On the peripheries of the lung the tumour is often invasive and stapled resection is preferred to attempting to preserve every last piece of lung as it will reduce air leak.
We prefer to leave the patient on prophylactic antibiotics for as long as the drains are in situ as the risk of pleural sepsis is increased by the presence of foreign material in the cavity.
The use of a biological prosthesis which is incorporated into the body’s own tissues may reduce the risk of infection seeding the patch and prolonging the sepsis and eventually requiring patch removal.
Tips
- Wear two pairs of gloves. Identify tissue planes by manual dissection, open planes by electrocautery onto double-gloved fingers;
- To reduce blood loss from the parenchymal surface during visceral pleurectomy it is advisable to isolate the main pulmonary artery and temporarily clamp the vessel to reduce pulmonary blood flow, if tolerated;
- In the case of bulkier tumours visceral pleurectomy is more straightforward but the parietal pleurectomy can be more challenging due to chest wall invasion;
- There may be the temptation to leave the pericardium widely open and avoid reconstruction. Unfortunately the preservation of the adjacent lung does not guard against cardiac dislocation and sudden loss of cardiac output.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wolf AS, Flores RM. Current Treatment of Mesothelioma: Extrapleural Pneumonectomy Versus Pleurectomy/Decortication. Thorac Surg Clin 2016;26:359-75. [Crossref] [PubMed]
- Hasegawa S. Extrapleural pneumonectomy or pleurectomy/decortication for malignant pleural mesothelioma. Gen Thorac Cardiovasc Surg 2014;62:516-21. [Crossref] [PubMed]
- Sharkey AJ, Tenconi S, Nakas A, et al. The effects of an intentional transition from extrapleural pneumonectomy to extended pleurectomy/decortication. Eur J Cardiothorac Surg 2016;49:1632-41. [Crossref] [PubMed]
- Rice D. Standardizing surgical treatment in malignant pleural mesothelioma. Ann Cardiothorac Surg 2012;1:497-501. [PubMed]
- Rusch VW. Pleurectomy and Decortication: How I Teach It. Ann Thorac Surg 2017;103:1374-7. [Crossref] [PubMed]
- Sharkey AJ, Bilancia R, Tenconi S, et al. The management of the diaphragm during radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2016;50:311-6. [Crossref] [PubMed]
Cite this article as: Di Martino M, Waller DA. Pleurectomy/decortication for malignant pleural mesothelioma. Shanghai Chest 2017;1:13.